Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors - PubMed (original) (raw)
Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors
F Ossendorp et al. J Exp Med. 1998.
Abstract
This study shows that induction of tumor-specific CD4+ T cells by vaccination with a specific viral T helper epitope, contained within a synthetic peptide, results in protective immunity against major histocompatibility complex (MHC) class II negative, virus-induced tumor cells. Protection was also induced against sarcoma induction by acutely transforming retrovirus. In contrast, no protective immunity was induced by vaccination with an unrelated T helper epitope. By cytokine pattern analysis, the induced CD4+ T cells were of the T helper cell 1 type. The peptide-specific CD4+ T cells did not directly recognize the tumor cells, indicating involvement of cross-priming by tumor-associated antigen-presenting cells. The main effector cells responsible for tumor eradication were identified as CD8+ cytotoxic T cells that were found to recognize a recently described immunodominant viral gag-encoded cytotoxic T lymphocyte (CTL) epitope, which is unrelated to the viral env-encoded T helper peptide sequence. Simultaneous vaccination with the tumor-specific T helper and CTL epitopes resulted in strong synergistic protection. These results indicate the crucial role of T helper cells for optimal induction of protective immunity against MHC class II negative tumor cells. Protection is dependent on tumor-specific CTLs in this model system and requires cross-priming of tumor antigens by specialized antigen-presenting cells. Thus, tumor-specific T helper epitopes have to be included in the design of epitope-based vaccines.
Figures
Figure 1
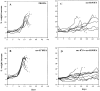
T helper peptide vaccination protects mice against intraperitoneal tumor induction. C57BL/6 mice were vaccinated with synthetic peptide (100 μg/mouse) in a 50% PBS/IFA (vol/vol) emulsion subcutaneously at day −14 and challenged with 1,000 RMA cells intraperitoneally at day zero. The weights of the individual mice were monitored every other day and the percent weight increase is depicted. Each line is an individual mouse. (A) Six mice were injected with an emulsion of IFA with PBS without peptide as a negative control. (B) Six mice were vaccinated with the subdominant Kb-presented MuLV env–gp70 CTL epitope peptide env-Kb8 (SSWDFITV). (C) Seven mice were vaccinated with the I-Ab–presented 19-mer MuLV env–gp70 T helper epitope peptide env-H19 (EPLTSLTPRCNTAWNRLKL). (D) 10 mice were vaccinated with a mixture of env-Kb8 and env-H19 (both 100 μg) in a single vaccine depot.
Figure 2
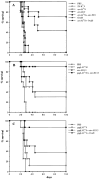
(A) Tumor-specific T helper peptide vaccination increases long-term survival of mice challenged with tumor cells. C57BL/6 mice were vaccinated with synthetic peptides as described in the legend of Fig. 1. All groups received IFA (50% vol/vol) with or without 100 μg peptide subcutaneously. The percentage of surviving mice is depicted. Mice were killed when the weight increase was >25%. PBS, nonimmunized control (n = 6); SV-Kb9, nonrelated Sendai virus nucleoprotein Kb-presented CTL epitope (FAPGNYPAL; n = 6); env-Kb8, MuLV env–gp70 subdominant CTL epitope (n = 7); env-H19, 19-mer MuLV env–gp70 T helper epitope (n = 7); env-Kb8 + env-H19, mixture vaccine of MuLV CTLs and T helper epitopes (n = 10); OvaH, nonrelated ovalbumin I-Ab–presented T helper epitope (ISQAVHAAHAEINEAGR; n = 6); env-Kb8 + OvaH, mixture of MuLV subdominant CTL epitope and ovalbumin T helper epitope. Significance of differences (log rank test): PBS versus env-H19: P = 0.04; PBS versus env-Kb8 + env-H19: P = 0.0015; env-H19 versus env-Kb8 + env-H19: P = 0.28 (not significant; n.s.). (B) Combination of T helper peptide and the immunodominant gag-L CTL epitope induces highly efficient tumor protection. C57BL/6 mice were immunized with synthetic peptides in IFA subcutaneously (50 μg/mouse) at day −14 and challenged with 103 RMA tumor cells intraperitoneally. Long-term survival was monitored. gag-L-Db10, gag-L CTL epitope (LCCLCLTVFL); env-H13.3, env-gp70 T helper peptide (SLTPRCNTAWNRL). All groups: n = 10. Significance of differences (log rank test): PBS versus gag-L-Db10: P = 0.0078; PBS versus env-H13.3: P = 0.0078; PBS versus gag-L-Db10 + env-H13.3: P = 0.0001; gag-L-Db10 versus gag-L-Db10 + env-H13.3: P = 0.0108; env-H13.3 versus gag-L-Db10 + env-H13.3: P = 0.025. (C) Efficient protection by dominant CTL epitope requires specific help. Similar experiment using the 9-mer dominant CTL epitope gag-L–Db9 in combination with either the MuLV T helper peptide env-H13.3 or the nonrelated OvaH T helper peptide. All groups: n = 8. Significance of differences (log rank test): gag-L-Db9 versus gag-L-Db9 + OvaH: P = 0.169 (n.s.); gag-L-Db9 versus gag-L-Db9 + env-H13.3: P = 0.003; gag-L-Db9 + OvaH versus gag-L-Db9 + env-H13.3: P = 0.04; PBS versus gag-L-Db9 + env-H13.3: P = 0.0004.
Figure 3
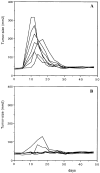
T helper peptide vaccination protects mice against retrovirus-induced sarcoma. C57BL/6 mice were vaccinated at day −14 with (A) PBS-IFA subcutaneously (n = 7) and (B) 13-mer MuLV T helper peptide H13.3 (SLTPRCNTAWNRL), 10 μg/mouse in IFA subcutaneously (n = 7). At day zero mice were injected in the left thigh muscle with 103 focus-forming units of MoMSV. Mice were monitored for tumor size using a caliper measuring two perpendicular diameters. Each line represents one individual mouse.
Figure 4
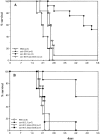
Both CD4+ and CD8+ T cells are involved in the protective mechanism. C57BL/6 mice were treated with anti-CD4 (GK1.5) and anti-CD8 (2.43) monoclonal antibodies leading to selective depletion (>95%) of these T cell subsets. (A) Mice were vaccinated with 100 μg of H19 in IFA subcutaneously (day −14) and treated five times with 100 μg GK1.5 monoclonal antibody in 200 μl PBS intraperitoneally during the vaccination period (days −14, −13, −10, −7, and −4) or as a control with PBS. At day 0, 103 RMA cells were injected intraperitoneally. Significance of differences (log rank test): PBS versus env-H19: P = 0.0002; env-H19 + anti-CD4 versus env-H19: P = 0.0009; PBS versus env-H19 + anti-CD4: P = 0.577 (n.s.). (B) Mice were vaccinated with 10 μg T helper peptide H13.3 in IFA subcutaneously at day −14 and 103 RMA cells were injected intraperitoneally at day 0. Antibody treatment (three times with 100 μg of GK1.5 or 2.43 intraperitoneally) was done during the effector phase (days 0, 3, and 7). Significance of differences (log rank test): PBS versus env-H13.3: P = 0.0027; env-H13.3/anti-CD4 versus env-H13.3: P = 0.0013; env-H13.3/anti-CD8 versus env-H13.3: P = 0.0068.
Figure 5
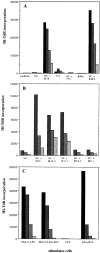
MuLV-specific CD4+ T cells can be isolated from T helper peptide-immunized mice. CD4+ T cell clones were isolated from T helper peptide-immunized and RMA-challenged mice and tested in a 3-d proliferation assay. Values represent means of triplicate measurements. (A) CD4+ clone 3A12 was isolated from a spleen of a protected mouse, 2 mo after tumor challenge, that was previously immunized with the 11-mer T helper H11.1 peptide. Spleen cells were in vitro restimulated with mitomycin-treated and irradiated RMA tumor cells and CD4+ T cell clones were isolated by limiting dilution. Shaded bars from black to gray: 4 × 104, 2 × 104, 104, and 5 × 103 cells/well, respectively. env-H19 peptide was used at 10 μg/ml. SC, irradiated spleen cells (105/well). RMA cells were mitomycin treated, irradiated, and used at 2 × 104 cells/well with or without irradiated spleen cells. 771 is a B cell lymphoma cell line isolated from a tumor induced by the AKV type MCF1233 MuLV that lacks the T helper epitope sequence in env (2 × 104 irradiated cells/well). (B) Peptide fine specificity of CD4+ clone 3A12. Peptides were used at 5 μg/ml. Shaded bars from dark to light gray: 2 × 104, 104, and 5 × 103 cells/well, respectively. (C) Moloney MuLV specificity of clone 3A12. LPS B cell blasts were induced using C57BL/6 spleen cells in the presence or absence of Moloney MuLV (MoLV-LPS). After 3 d, the LPS blasts were isolated, irradiated, and used as stimulator cells (LPS, 105 cells/well) for clone 3A12 in the presence or absence of H19 peptide (10 μg/ml).
Figure 6
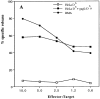
Cytotoxic T cells isolated from protected mice recognize the immunodominant MuLV gag-L peptide. CD8+ T cell bulk cultures were isolated from spleen cells of a C57BL/6 mouse that was immunized with the H11.1 T helper peptide and survived RMA tumor challenge. 2H9 was a representative clone isolated from this RMA-specific bulk culture by limiting dilution. (A) Cytotoxicity assay using RMA cells and HeLa cells selectively transfected with the MHC class I Db molecule as target cells. HeLaDb cells were incubated with the gag-L (CCLCLTVFL) peptide (1 μg/ml) during the assay. (B) No reactivity of 2H9 CTL clone with the T helper peptide sequence. EL-4 cells (H-2b) were incubated with different synthetic peptides (1 μg/ml). gag-L–Db9 is the dominant gag-L epitope CCLCLTVFL; gag-L–SIV9, another upstream gag-leader sequence reported to comprise a CTL epitope SIVLCCLCL (31).
Figure 6
Cytotoxic T cells isolated from protected mice recognize the immunodominant MuLV gag-L peptide. CD8+ T cell bulk cultures were isolated from spleen cells of a C57BL/6 mouse that was immunized with the H11.1 T helper peptide and survived RMA tumor challenge. 2H9 was a representative clone isolated from this RMA-specific bulk culture by limiting dilution. (A) Cytotoxicity assay using RMA cells and HeLa cells selectively transfected with the MHC class I Db molecule as target cells. HeLaDb cells were incubated with the gag-L (CCLCLTVFL) peptide (1 μg/ml) during the assay. (B) No reactivity of 2H9 CTL clone with the T helper peptide sequence. EL-4 cells (H-2b) were incubated with different synthetic peptides (1 μg/ml). gag-L–Db9 is the dominant gag-L epitope CCLCLTVFL; gag-L–SIV9, another upstream gag-leader sequence reported to comprise a CTL epitope SIVLCCLCL (31).
Similar articles
- FBL-reactive CD8+ cytotoxic and CD4+ helper T lymphocytes recognize distinct Friend murine leukemia virus-encoded antigens.
Klarnet JP, Kern DE, Okuno K, Holt C, Lilly F, Greenberg PD. Klarnet JP, et al. J Exp Med. 1989 Feb 1;169(2):457-67. doi: 10.1084/jem.169.2.457. J Exp Med. 1989. PMID: 2562982 Free PMC article. - Tumour-specific CTL response requiring interactions of four different cell types and recognition of MHC class I and class II restricted tumour antigens.
Schirrmacher V, Schild HJ, Gückel B, von Hoegen P. Schirrmacher V, et al. Immunol Cell Biol. 1993 Aug;71 ( Pt 4):311-26. doi: 10.1038/icb.1993.36. Immunol Cell Biol. 1993. PMID: 7901150 - Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor.
Tomita Y, Yuno A, Tsukamoto H, Senju S, Kuroda Y, Hirayama M, Irie A, Kawahara K, Yatsuda J, Hamada A, Jono H, Yoshida K, Tsunoda T, Kohrogi H, Yoshitake Y, Nakamura Y, Shinohara M, Nishimura Y. Tomita Y, et al. Clin Cancer Res. 2013 Aug 15;19(16):4508-20. doi: 10.1158/1078-0432.CCR-13-0197. Epub 2013 May 28. Clin Cancer Res. 2013. PMID: 23714729 - Lessons from T cell responses to virus induced tumours for cancer eradication in general.
Melief CJ, Kast WM. Melief CJ, et al. Cancer Surv. 1992;13:81-99. Cancer Surv. 1992. PMID: 1423326 Review.
Cited by
- Divergent HLA variations and heterogeneous expression but recurrent HLA loss-of- heterozygosity and common HLA-B and TAP transcriptional silencing across advanced pediatric solid cancers.
Lim WC, Marques Da Costa ME, Godefroy K, Jacquet E, Gragert L, Rondof W, Marchais A, Nhiri N, Dalfovo D, Viard M, Labaied N, Khan AM, Dessen P, Romanel A, Pasqualini C, Schleiermacher G, Carrington M, Zitvogel L, Scoazec JY, Geoerger B, Salmon J. Lim WC, et al. Front Immunol. 2024 Jan 22;14:1265469. doi: 10.3389/fimmu.2023.1265469. eCollection 2023. Front Immunol. 2024. PMID: 38318504 Free PMC article. - IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age.
Tsukamoto H, Senju S, Matsumura K, Swain SL, Nishimura Y. Tsukamoto H, et al. Nat Commun. 2015 Apr 7;6:6702. doi: 10.1038/ncomms7702. Nat Commun. 2015. PMID: 25850032 Free PMC article. - Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells.
Lu X, Ding ZC, Cao Y, Liu C, Habtetsion T, Yu M, Lemos H, Salman H, Xu H, Mellor AL, Zhou G. Lu X, et al. J Immunol. 2015 Feb 15;194(4):2011-21. doi: 10.4049/jimmunol.1401894. Epub 2015 Jan 5. J Immunol. 2015. PMID: 25560408 Free PMC article. - Adequate antigen availability: a key issue for novel approaches to tumor vaccination and tumor immunotherapy.
Accolla RS, Tosi G. Accolla RS, et al. J Neuroimmune Pharmacol. 2013 Mar;8(1):28-36. doi: 10.1007/s11481-012-9423-7. Epub 2012 Dec 7. J Neuroimmune Pharmacol. 2013. PMID: 23224729 Review. - MDM2 inhibitors in cancer immunotherapy: Current status and perspective.
Zeng Q, Zeng S, Dai X, Ding Y, Huang C, Ruan R, Xiong J, Tang X, Deng J. Zeng Q, et al. Genes Dis. 2024 Mar 28;11(6):101279. doi: 10.1016/j.gendis.2024.101279. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39263534 Free PMC article. Review.
References
- Ostrand-Rosenberg S, Thakur A, Clements V. Rejection of mouse sarcoma cells after transfection of MHC class II genes. J Immunol. 1990;144:4068–4071. - PubMed
- Chen P, Aanathaswamy H. Rejection of K1735 murine melanoma in syngeneic hosts requires expression of MHC class I antigens and either class II antigens or IL-2. J Immunol. 1993;151:244–255. - PubMed
- Yoshimura A, Shiku H, Nakayama E. Rejection of an IA+ variant line of FBL-3 leukemia by cytotoxic T lymphocytes with CD4+ and CD4−CD8−T cell receptor-αβ phenotypes generated in CD8-depleted C57BL/6 mice. J Immunol. 1993;150:4900–4910. - PubMed
- Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials