Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions - PubMed (original) (raw)
Review
Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions
S D Novakovic et al. J Neurosci. 1998.
Abstract
The novel sodium channel PN3/alpha-SNS, which was cloned from a rat dorsal root ganglion (DRG) cDNA library, is expressed predominantly in small sensory neurons and may contribute to the tetrodotoxin-resistant (TTXR) sodium current that is believed to be associated with central sensitization in chronic neuropathic pain states. To assess further the role of PN3, we have used electrophysiological, in situ hybridization and immunohistochemical methods to monitor changes in TTXR sodium current and the distribution of PN3 in normal and peripheral nerve-injured rats. (1) Whole-cell patch-clamp recordings showed that there were no significant changes in the TTXR and TTX-sensitive sodium current densities of small DRG neurons after chronic constriction injury (CCI) of the sciatic nerve. (2) Additionally, in situ hybridization showed that there was no change in the expression of PN3 mRNA in the DRG up to 14 d after CCI. PN3 mRNA was not detected in sections of brain and spinal cord taken from either normal or nerve-injured rats. (3) In contrast, immunohistochemical studies showed that major changes in the subcellular distribution of PN3 protein were caused by either CCI or complete transection of the sciatic nerve. The intensity of PN3 immunolabeling decreased in small DRG neurons and increased in sciatic nerve axons at the site of injury. The alteration in immunolabeling was attributed to translocation of presynthesized, intracellularly located PN3 protein from neuronal somata to peripheral axons, with subsequent accumulation at the site of injury. The specific subcellular redistribution of PN3 after peripheral nerve injury may be an important factor in establishing peripheral nerve hyperexcitability and resultant neuropathic pain.
Figures
Fig. 1.
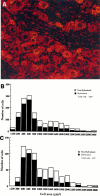
Isolation of TTXR and TTXSsodium currents. A, Total, TTXR, and TTXS sodium currents were recorded from a single DRG neuron taken from a nerve-injured rat. Sodium currents were evoked by stepping from a holding potential of −100 mV to a range of test potentials (−90 to +40 mV) for 40 msec. Currents were recorded before and 5 min after application of 1 μ
m
TTX to the cell.B, I_–_V and_G_–V relationships were plotted for total, TTXR, and TTXS sodium currents (same neuron as in A).
Fig. 2.
Radioactive in situ hybridization showing distribution of PN3 mRNA in the rat DRG neurons.A, Normal DRG tissue, 10-μm-thick cryosections. Emulsion autoradiography of the section hybridized with antisense probe. PN3 mRNA is localized predominantly in small DRG neurons, and strong cytoplasmic signals could be observed. Some large neurons have low-level labeling. Cell nuclei are not labeled. B, Distribution of hybridization by cell size in the normal DRG tissue.C, Distribution of hybridization in the CCI tissue. No significant differences were observed.
Fig. 3.
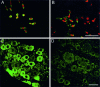
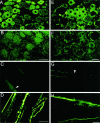
Immunocytochemistry of PN3 transfected cells and normal DRG tissue. A, B, Immunolabeling of PN3 transfected and nontransfected CHL cells was performed with specific antiserum as a measure of antibody specificity. Transfected cells are brightly labeled (A, green) when compared with nontransfected cells (B, green). Propidium iodide was used for labeling of cell nuclei (red). Scale bar, 25 μm. C, D, Immunofluorescence labeling of normal DRG tissue. In agreement with in situ hybridization (Fig.2), small neurons exhibit a bright fluorescent signal (C, green), compared with low-level labeling in large neurons and control with preabsorbed antibodies (D). Scale bar, 50 μm.
Fig. 4.
PN3 immunolabeling of brain and heart tissue. No specific PN3 labeling was detected in rat brain (A) or heart tissue (B) in the experimental conditions that we used (background labeling was identical with the preabsorbed control, not shown), thus confirming the exclusive localization of PN3 to the peripheral nervous system. It also demonstrated that PN3 antibody (20075) did not cross-react with any other sodium channel isoforms. Scale bar: A, 200 μm;B, 50 μm.
Fig. 5.
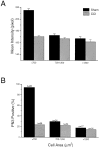
Quantification of immunolabeling in normal DRG.A, Mean intensity of immunolabeling according to cell size. B, Percentage of PN3-positive neurons according to cell size.
Fig. 6.
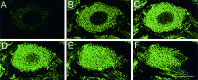
Confocal microscopy of PN3-labeled DRG cells.A–F, Six optical sections (at 1.8 μm intervals) from a 20-μm-thick cryosection labeled with PN3 antiserum. This illustrates that most immunolabeling is located intracellularly. Scale bar, 10 μm.
Fig. 7.
Immunolabeling of spinal cord section as revealed by immunoperoxidase–DAB. A, Specific PN3 labeling can be observed in the superficial laminae of the spinal cord dorsal horn.B, No labeling can be observed in preabsorbed antibody control. Scale bar, 100 μm.
Fig. 8.
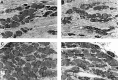
Immunocytochemistry (peroxidase–DAB) of DRG cells from sham-operated and CCI animals. A, Tissue from sham-operated animals revealed a similar labeling pattern as tissue from normal animals (shown in Fig. 3 by immunofluorescence), 7 d PS. B, CCI tissue, 7 d PS. Small cells have lost some of the high-intensity labeling. C, CCI tissue, 14 d PS. In contrast to tissue from sham-operated animals, immunolabeling is of the same intensity in small and large DRG cells.D, CCI, 28 d PS. The normal immunolabeling pattern reappears, with small cells regaining their high intensity labeling. Scale bar, 50 μm.
Fig. 9.
Mean labeling intensity and percentage of PN3-positive neurons in DRG taken from CCI and sham-operated animals, 14 d PS. A, Mean intensity of labeling in small (<700 μm2), medium (700–1200 μm2), and large neurons (>1200 μm2) taken from sham-operated (black bars) and CCI animals (gray bars). There is a significant difference in the intensity of labeling of small neurons from the two groups of animals. There is no significant difference in the intensity of labeling of medium and large neurons.B, Percentage of PN3-positive neurons in sham-operated (black bars) and CCI animals (gray bars). The percentage of small neurons positive for PN3 is significantly lower in tissue from CCI animals. The percentage of PN3-positive medium and large neurons does not differ significantly.
Fig. 10.
Immunofluorescence of CCI DRG cell somata and their peripheral axons. A–D represent immunolabeling with antiserum 20075, and E–H show almost identical results reproduced with antiserum 20073. Fluorescent labeling of DRG tissue taken from sham-operated animals (A, E), 14 d PS, which is the same as in normal tissue. In tissue from CCI animals, there is a dramatic reduction in labeling intensity in small cells (B, F), 14 d PS. Scale bar, 50 μm.C and G represent fluorescent labeling of teased axons from normal sciatic nerve (arrows show unlabeled nodes of Ranvier). In axons from CCI animals, there is intense labeling (D, H). Scale bar, 25 μm.
Fig. 11.
Immunohistochemistry (peroxidase–DAB) of DRG tissue from sham-operated and nerve-transected animals.A, Tissue section from sham-operated animal, 5 d PS. B, Section from animal with nerve transection, 5 d PS. C, Section from animal with nerve transection, 21 d PS. Scale bar, 50 μm.
Similar articles
- Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons.
Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Fjell J, et al. Brain Res Mol Brain Res. 1999 Apr 20;67(2):267-82. doi: 10.1016/s0169-328x(99)00070-4. Brain Res Mol Brain Res. 1999. PMID: 10216225 - Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy.
Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA. Sleeper AA, et al. J Neurosci. 2000 Oct 1;20(19):7279-89. doi: 10.1523/JNEUROSCI.20-19-07279.2000. J Neurosci. 2000. PMID: 11007885 Free PMC article. - Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo.
Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Dib-Hajj SD, et al. J Neurophysiol. 1998 May;79(5):2668-76. doi: 10.1152/jn.1998.79.5.2668. J Neurophysiol. 1998. PMID: 9582237 - A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain.
Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, Kassotakis L, Novakovic S, Rabert DK, Sangameswaran L, Hunter JC. Porreca F, et al. Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):7640-4. doi: 10.1073/pnas.96.14.7640. Proc Natl Acad Sci U S A. 1999. PMID: 10393873 Free PMC article. Review. - Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain.
Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Waxman SG, et al. Muscle Nerve. 1999 Sep;22(9):1177-87. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. Muscle Nerve. 1999. PMID: 10454712 Review.
Cited by
- Expression of activating transcription factor 3 (ATF3) in uninjured dorsal root ganglion neurons in a lower trunk avulsion pain model in rats.
Matsuura Y, Ohtori S, Iwakura N, Suzuki T, Kuniyoshi K, Takahashi K. Matsuura Y, et al. Eur Spine J. 2013 Aug;22(8):1794-9. doi: 10.1007/s00586-013-2733-5. Epub 2013 Mar 8. Eur Spine J. 2013. PMID: 23471575 Free PMC article. - Involvement of Nav 1.8 sodium ion channels in the transduction of mechanical pain in a rodent model of osteoarthritis.
Schuelert N, McDougall JJ. Schuelert N, et al. Arthritis Res Ther. 2012 Jan 7;14(1):R5. doi: 10.1186/ar3553. Arthritis Res Ther. 2012. PMID: 22225591 Free PMC article. - The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice.
Roza C, Laird JM, Souslova V, Wood JN, Cervero F. Roza C, et al. J Physiol. 2003 Aug 1;550(Pt 3):921-6. doi: 10.1113/jphysiol.2003.046110. Epub 2003 Jun 24. J Physiol. 2003. PMID: 12824446 Free PMC article. - The sodium channel blocker RS100642 reverses down-regulation of the sodium channel alpha-subunit Na(v) 1.1 expression caused by transient ischemic brain injury in rats.
Yao C, Williams AJ, Lu XC, Price RA, Cunningham BS, Berti R, Tortella FC, Dave JR. Yao C, et al. Neurotox Res. 2003;5(4):245-53. doi: 10.1007/BF03033382. Neurotox Res. 2003. PMID: 12835116 - Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes.
Theile JW, Cummins TR. Theile JW, et al. Front Pharmacol. 2011 Oct 4;2:54. doi: 10.3389/fphar.2011.00054. eCollection 2011. Front Pharmacol. 2011. PMID: 22007172 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Arbuckle JB, Docherty RJ. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995;185:70–73. - PubMed
- Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for pain-related behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. - PubMed
- Basbaum AI, Gautron M, Jazat F, Mayes M, Guilbaud G. The spectrum of fiber loss in a model of neuropathic pain in the rat: an electron microscopic study. Pain. 1991;47:359–367. - PubMed
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases