Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus - PubMed (original) (raw)
Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus
Y Chen et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Cubitus interruptus (Ci) is a transcriptional factor that is positively regulated by the hedgehog (hh) signaling pathway. Recent work has shown that a 75-kDa proteolytic product of the full-length CI protein translocates to the nucleus and represses the transcription of CI target genes. In cells that receive the hh signal, the proteolysis of CI is inhibited and the full-length protein can activate the hh target genes. Because protein kinase A (PKA) inhibits the expression of the hh target genes in developing embryos and discs and the loss of PKA activity results in elevated levels of full-length CI protein, PKA might be involved directly in the regulation of CI proteolysis. Here we demonstrate that the PKA pathway antagonizes the hh pathway by phosphorylating CI. We show that the PKA-mediated phosphorylation of CI promotes its proteolysis from the full-length active form to the 75-kDa repressor form. The PKA catalytic subunit increases the proteolytic processing of CI and the PKA inhibitor, PKI, blocks the processing. In addition, cells do not process the CI protein to the 75-kDa repressor when all of the PKA sites in CI are mutated. Mutant CI proteins that cannot be phosphorylated by PKA have increased transcriptional activity compared with wild-type CI. In addition, exogenous PKA can increase further the transcriptional activity of the CI mutant, suggesting that PKA has a second positive, indirect effect on CI activity. In summary, we show that the modulation of the hh signaling pathway by PKA occurs directly at the level of CI phosphorylation.
Figures
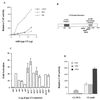
Figure 1
Effect of PKA phosphorylation on CI-mediated transcriptional activation. (A) One hundred nanograms of pPac-luciferase, 5 μg of the ADHCAT/GLI6BS reporter gene, 4 μg pPac-PKA or pPac-PKI, and an increasing amount (0–12 μg) of pPac-CI (wild type) were transfected into Kc cells. Four micrograms of PKA and PKI represent the amount of DNA that gives maximal activities in the dose response curves (data not shown). (B) Schematic diagram showing the structure of CI protein. (C) Transfections were carried out by using 100 ng of pPac-luciferase, 5 μg of the ADHCAT/GLI6BS reporter gene, and 2 μg of the different PKA mutants. (D) Transfections were performed as in A by using 2 μg of pPac-CI (wild type) and 2 μg of pPac-CI (null). CAT activities were normalized to the corresponding luciferase activities. Data represent means ± SEM.
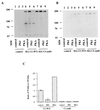
Figure 2
Effect of PKA phosphorylation on CI proteolysis. (A) A mixture of 10 μg of pPac (lanes 1–3) or pPac-HA-CI (WT) (lanes 4–6) or pPac-HA-CI (null) (lanes 7–9), and 10 μg of pPac (lanes 1, 4, and 7), pPac-PKI (lanes 2, 5, and 8), or pPac-PKA (lanes 3, 6, and 9) were transfected into Kc cells. HA-tagged CI proteins were immunoprecipitated with a rat monoclonal anti-HA antibody and probed with a mouse anti-HA antibody. (B) The filter in A was probed with a rat anti-CI antibody. In other experiments, the level of full-length CI is higher in the PKI lane than illustrated here and we attribute the difference to the variation often associated with stripping and reprobing. (C) Intensity ratio of 75–155 kDa plotted according to A. Because enhanced chemiluminescence signals can be nonlinear, the data represent scans of the mid-range exposures from two separate experiments. They include the means ± SD.
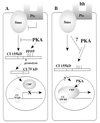
Figure 3
Schematic drawing showing the relationship of PKA and hh pathways on CI proteolysis and activity. (A) In a cell that is not stimulated by the hh signaling pathway, PKA phosphorylates cytoplasmic CI. The phospho-CI is a target for proteolysis and the 75-kDa protein enters the nucleus and represses the CI target genes, perhaps blocking the recruitment of dCBP to the promoter. (B) In a cell that receives the hh signal, the phosphorylation of CI by PKA in the cytoplasm is inhibited and CI is not proteolysed to the 75-kDa repressor. This allows the active form of CI to enter the nucleus, recruit dCBP to the promoter, and activate the CI target genes. The CI activation of transcription is potentiated by PKA.
Similar articles
- Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A.
Price MA, Kalderon D. Price MA, et al. Development. 1999 Oct;126(19):4331-9. doi: 10.1242/dev.126.19.4331. Development. 1999. PMID: 10477300 - Mutants of cubitus interruptus that are independent of PKA regulation are independent of hedgehog signaling.
Chen Y, Cardinaux JR, Goodman RH, Smolik SM. Chen Y, et al. Development. 1999 Aug;126(16):3607-16. doi: 10.1242/dev.126.16.3607. Development. 1999. PMID: 10409506 - Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus.
Wang G, Wang B, Jiang J. Wang G, et al. Genes Dev. 1999 Nov 1;13(21):2828-37. doi: 10.1101/gad.13.21.2828. Genes Dev. 1999. PMID: 10557210 Free PMC article. - Hedgehog signalling: Ci complex cuts and clasps.
Kalderon D. Kalderon D. Curr Biol. 1997 Dec 1;7(12):R759-62. doi: 10.1016/s0960-9822(06)00398-8. Curr Biol. 1997. PMID: 9382827 Review. - Decoding Ci: from partial degradation to inhibition.
Xiong Y, Liu C, Zhao Y. Xiong Y, et al. Dev Growth Differ. 2015 Feb;57(2):98-108. doi: 10.1111/dgd.12187. Epub 2014 Dec 14. Dev Growth Differ. 2015. PMID: 25495033 Review.
Cited by
- Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing.
Wang B, Li Y. Wang B, et al. Proc Natl Acad Sci U S A. 2006 Jan 3;103(1):33-8. doi: 10.1073/pnas.0509927103. Epub 2005 Dec 21. Proc Natl Acad Sci U S A. 2006. PMID: 16371461 Free PMC article. - Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus.
Merchant M, Evangelista M, Luoh SM, Frantz GD, Chalasani S, Carano RA, van Hoy M, Ramirez J, Ogasawara AK, McFarland LM, Filvaroff EH, French DM, de Sauvage FJ. Merchant M, et al. Mol Cell Biol. 2005 Aug;25(16):7054-68. doi: 10.1128/MCB.25.16.7054-7068.2005. Mol Cell Biol. 2005. PMID: 16055717 Free PMC article. - Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube.
Tuson M, He M, Anderson KV. Tuson M, et al. Development. 2011 Nov;138(22):4921-30. doi: 10.1242/dev.070805. Epub 2011 Oct 17. Development. 2011. PMID: 22007132 Free PMC article. - Genetic evidence for a protein kinase A/cubitus interruptus complex that facilitates processing of cubitus interruptus in Drosophila.
Kiger JA Jr, O'Shea C. Kiger JA Jr, et al. Genetics. 2001 Jul;158(3):1157-66. doi: 10.1093/genetics/158.3.1157. Genetics. 2001. PMID: 11454764 Free PMC article. - Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development.
Pan Y, Wang C, Wang B. Pan Y, et al. Dev Biol. 2009 Feb 1;326(1):177-89. doi: 10.1016/j.ydbio.2008.11.009. Epub 2008 Nov 20. Dev Biol. 2009. PMID: 19056373 Free PMC article.
References
- Nusslein-Volhard C, Wieschaus E. Nature (London) 1980;287:795–801. - PubMed
- Lee J J, von Kessler D P, Parks S, Beachy P A. Cell. 1992;71:33–50. - PubMed
- Heemskerk J, DiNardo S. Cell. 1994;76:449–460. - PubMed
- Kojima T, Michiue T, Orihara M, Saigo K. Gene. 1994;148:221–217. - PubMed
- Mohler J, Vani K. Development (Cambridge, UK) 1992;115:957–971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases