CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune responses - PubMed (original) (raw)
CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune responses
H T Maecker et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Mice lacking CD81 (TAPA-1), a widely expressed tetraspanin molecule, have impaired antibody responses to protein antigens. This defect is specific to antigens that preferentially stimulate a T helper 2 response (ovalbumin or keyhole limpet hemocyanin in alum) and is only seen with T cell-dependent antigens. Absence of CD81 on B cells is sufficient to cause the defect. Also, antigen-specific interleukin (IL) 4 production is greatly reduced in the spleen and lymph nodes of CD81-null mice compared with heterozygous littermates. Thus, expression of CD81 on B cells is critical for inducing optimal IL-4 and antibody production during T helper 2 responses. These findings suggest that CD81 may interact with a ligand on T cells to signal IL-4 production. By using a soluble form of CD81 as a probe, a putative ligand for CD81 was identified on a subset of B and T cells. Two possible models for the interaction of CD81 on B cells with a potential ligand on either B or T cells are proposed.
Figures
Figure 1
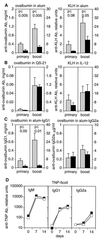
(A–C) Response of CD81-null mice to T dependent antigens varies with adjuvant. CD81-null mice (solid bars) and heterozygous littermates (hatched bars) were immunized i.p. with ovalbumin or KLH either precipitated in alum or given with QS-21 or IL-12 as adjuvants. Antigen-specific serum antibody responses were measured 12 weeks after a single immunization (primary) or 18 days after a second injection (boost). (A) Responses of CD81-null mice were significantly lower than heterozygotes to ovalbumin or KLH in alum, which induces a Th2 response. (B) Responses of CD81-null mice were not significantly different from heterozygotes when immunized with ovalbumin in QS-21 or KLH in IL-12, which stimulate a Th1 response. (C) The IgG1 response to ovalbumin in alum was significantly decreased in CD81-null mice. IgG2a responses to ovalbumin in alum were not significantly different between groups. (D) Response of CD81-null mice to a T independent antigen. CD81-null mice (▪) and heterozygous littermates (□) were immunized i.p. with 25 μg of TNP-Ficoll. The serum antibody response to TNP was measured by ELISA at 7 and 14 days. There were no significant differences between groups. Data are the means ± SEM (n = 3 to 9 animals per group).
Figure 2
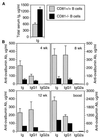
Chimeric mice were constructed by injection of CD81−/− ES cells (solid bars) or CD81+/+ ES cells (hatched bars) into blastocysts from B cell-deficient mice. (A) Total serum Ig of chimeric mice at 12 weeks of age. (B) Response of chimeric mice to ovalbumin in alum. Chimeric mice were immunized with ovalbumin in alum, and serum anti-ovalbumin antibody was measured at 4, 8, and 12 weeks after a single injection. Mice were then given a booster injection and secondary responses were measured 18 days later. Error bars indicate the SEM (n = 2 to 10 animals per group).
Figure 3
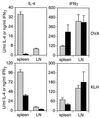
Antigen-specific IL-4 and IFN-γ responses in CD81-null mice (solid bars) and heterozygous littermates (hatched bars). Mice were immunized three times with the specified antigen in alum. Spleens and peripheral lymph nodes were removed 4 days after the last injection. Mononuclear cells were restimulated with ovalbumin or KLH for 3 days, then supernatants were harvested, and cytokines measured by ELISA (25). Bars indicate the SD of triplicate cultures.
Figure 4
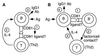
Schematic models for the role of CD81 in Th2 responses. In the simplest model (A), CD81 on B cells interacts directly with a putative ligand on T cells, stimulating IL-4 production, which leads to IgG1 antibody production. The interacting T cells expressing CD81 ligand may be those already committed to Th2 development, or they may be recruited into this pathway upon interaction with B cells expressing CD81. In a slightly different model (B), B cells interact first with a subset of other B cells expressing the putative CD81 ligand. These B cells then interact with T cells via cell contact and/or soluble factors to induce IL-4 secretion and IgG1 antibody production.
Similar articles
- Function of the tetraspanin molecule CD81 in B and T cells.
Levy S. Levy S. Immunol Res. 2014 May;58(2-3):179-85. doi: 10.1007/s12026-014-8490-7. Immunol Res. 2014. PMID: 24522698 Review. - Critical role of CD81 in cognate T-B cell interactions leading to Th2 responses.
Deng J, Dekruyff RH, Freeman GJ, Umetsu DT, Levy S. Deng J, et al. Int Immunol. 2002 May;14(5):513-23. doi: 10.1093/intimm/14.5.513. Int Immunol. 2002. PMID: 11978781 - Normal lymphocyte development but delayed humoral immune response in CD81-null mice.
Maecker HT, Levy S. Maecker HT, et al. J Exp Med. 1997 Apr 21;185(8):1505-10. doi: 10.1084/jem.185.8.1505. J Exp Med. 1997. PMID: 9126932 Free PMC article. - Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity.
van Zijverden M, van der Pijl A, Bol M, van Pinxteren FA, de Haar C, Penninks AH, van Loveren H, Pieters R. van Zijverden M, et al. Toxicol Appl Pharmacol. 2000 Oct 15;168(2):131-9. doi: 10.1006/taap.2000.9013. Toxicol Appl Pharmacol. 2000. PMID: 11032768 - CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system.
Levy S, Todd SC, Maecker HT. Levy S, et al. Annu Rev Immunol. 1998;16:89-109. doi: 10.1146/annurev.immunol.16.1.89. Annu Rev Immunol. 1998. PMID: 9597125 Review.
Cited by
- Autoimmunity and extrahepatic manifestations in treatment-naïve children with chronic hepatitis C virus infection.
Indolfi G, Bartolini E, Olivito B, Azzari C, Resti M. Indolfi G, et al. Clin Dev Immunol. 2012;2012:785627. doi: 10.1155/2012/785627. Epub 2012 May 8. Clin Dev Immunol. 2012. PMID: 22645623 Free PMC article. Review. - Function of the tetraspanin molecule CD81 in B and T cells.
Levy S. Levy S. Immunol Res. 2014 May;58(2-3):179-85. doi: 10.1007/s12026-014-8490-7. Immunol Res. 2014. PMID: 24522698 Review. - The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro T-cell proliferative responses.
Tarrant JM, Groom J, Metcalf D, Li R, Borobokas B, Wright MD, Tarlinton D, Robb L. Tarrant JM, et al. Mol Cell Biol. 2002 Jul;22(14):5006-18. doi: 10.1128/MCB.22.14.5006-5018.2002. Mol Cell Biol. 2002. PMID: 12077330 Free PMC article. - CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency.
van Zelm MC, Smet J, Adams B, Mascart F, Schandené L, Janssen F, Ferster A, Kuo CC, Levy S, van Dongen JJ, van der Burg M. van Zelm MC, et al. J Clin Invest. 2010 Apr;120(4):1265-74. doi: 10.1172/JCI39748. Epub 2010 Mar 8. J Clin Invest. 2010. PMID: 20237408 Free PMC article. - EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells.
Charrin S, Le Naour F, Labas V, Billard M, Le Caer JP, Emile JF, Petit MA, Boucheix C, Rubinstein E. Charrin S, et al. Biochem J. 2003 Jul 15;373(Pt 2):409-21. doi: 10.1042/BJ20030343. Biochem J. 2003. PMID: 12708969 Free PMC article.
References
- Nakamura T, Kamogawa Y, Bottomly K, Flavell R A. J Immunol. 1997;158:1085–1094. - PubMed
- Szabo S J, Jacobson N G, Dighe A S, Gubler U, Murphy K M. Immunity. 1995;2:665–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials