Vaccination with an attenuated Creutzfeldt-Jakob disease strain prevents expression of a virulent agent - PubMed (original) (raw)
Vaccination with an attenuated Creutzfeldt-Jakob disease strain prevents expression of a virulent agent
L Manuelidis. Proc Natl Acad Sci U S A. 1998.
Abstract
Although slow and persistent viruses often escape host defenses infection may be prevented by live vaccines. To determine whether an attenuated "slow" strain of the Creutzfeldt-Jakob disease agent (SY) could block expression of a virulent "fast" strain (FU), outbred CD-1 mice were inoculated intracerebrally with low infectious doses of SY and challenged 80 days later with higher doses of FU. For comparison, the same SY and FU samples were inoculated in two parallel control groups. All 18 superinfected mice showed incubation times identical to those inoculated with only the SY strain, yielding clinical disease >110 days later than predicted for the FU strain. Neurological signs, such as scratching and an extended clinical phase, were also characteristic for SY but not FU infection. Moreover, the widespread cortical pathology of FU was not detectable in superinfected mice. Western blot analyses further showed no strain-specific differences in prion protein (PrP) band profiles for all experimental groups, although there was approximately 10-fold more protease-resistant PrP (PrP-res) in FU brains during terminal disease. In contrast, infectivity assays revealed an approximately 10,000-fold difference between SY and FU at terminal stages, indicating that PrP-res content does not correlate with infectivity. In summary, an attenuated strain of the Creutzfeldt-Jakob disease agent evokes substantial interference against a virulent agent. Because superinfected mice had little PrP-res just before the onset of clinical disease and retained abundant cellular PrP, cellular PrP was not the factor limiting FU replication. The mechanisms underlying SY interference are not understood but could be based on host recognition of foreign molecular features shared by this class of invasive agents involving antibody production, and possibly involve defective viral particles produced by attenuated variants.
Figures
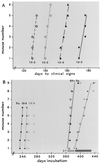
Figure 1
(A) Interval to clinical disease of FU homogenates (10−1) in serial 1:10 dilutions. Day 1 is the inoculation day. Lines indicate the best fit in each experimental group, where the incubation in every inoculated mouse is shown by a single point. As derived (31), the effective doubling time _t_i = [ln 2/ln(titer end/titer start)] × the interval in days. Thus, for example, the 45-day interval for a 1:1,000 dilution of FU yields a _t_i of 4.5 days. (B) Predicted times for FU clinical and terminal disease at 10−4 (diamonds with solid and dotted lines, respectively) and at 10−5 (large and small open circles, respectively). Horizontal bars at right show the range of all control mice inoculated with SY only at 10−2 (light for clinical signs and dark for terminal illness). SY vaccinated mice challenged with a dilution of 10−4 (5,000 IU) of FU at clinical and terminal disease (large and small solid triangles respectively) and with FU diluted 10−5 (large and small open triangles) are within the range of SY controls, with the best-fit lines for the SY + FU 10−4 mice at clinical (solid line) and more terminal (dotted line) illness shown. Although some superinfected mice were sacrificed earlier during the clinical phase, the median distance between solid and dashed lines in superinfected mice was significantly longer (P < 0.001) than in FU controls.
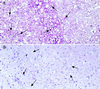
Figure 2
Typical sections of cerebral cortex from an FU mouse (A) and an SY mouse (B) challenged with 5,000 IU of FU, stained, and developed in parallel for PrP-res (red) as described (32). (A) Extreme vacuolization of the cerebral cortex and a diffuse red blush of PrP-res and punctate PrP-res deposits characteristic for FU (arrows) are shown. (B) Representative region of SY cortex at identical magnification with markedly fewer vacuoles (arrows). As in SY control cortex, no background blush of PrP-res or punctate deposits are seen. Section was hematoxylin-counterstained (blue) to show nuclei. Both sections are from the formalin-fixed inoculated half brain and other side was used for Western blotting (Fig. 3).
Figure 3
Chemiluminesent detection of PrP in brain homogenates (equal sample loads) from control and superinfected mice. The arrows at the top indicate the mouse samples from SY or FU infection or both. The PK arrow indicates that samples 1–10 (but not 11) were digested with proteinase K. Nl is from an uninfected mouse showing no PrP-res. Lane 11 shows a pool of four FU brains used to quantitate total PrP (the 100% reference) and taken from the same vial used for inoculation; undigested samples of SY showed comparable levels of total PrP (data not shown). The percent of total PrP-res and the days when each individual mouse was sacrificed are indicated below each lane. The FU control inoculum shows more PrP-res (lane 1) than any terminal interference mouse (lanes 2, 3, and 5–7). PrP-res is similarly lower in a representative parallel SY control mouse from this passage (lane 8) and in a pool of four brains from the subsequent SY serial passage (lane 9). The average PrP-res of individual superinfected brains was 4.1%, which is in good accord with the SY controls. Lane 4 shows the very minimal PrP-res levels in a random superinfected mouse late in incubation (340 days) but just before the onset of clinical symptoms. For quantitation, homogenates were digested for maximal PrP-res detection as described (31), and films in the linear range were analyzed by 16-bit densitometry on Western blots as described with both densitometry and autoradiographic standards (32). No residual PrP is seen at the slot (s) and molecular weight markers are indicated at left in kDa.
Similar articles
- Attenuated Creutzfeldt-Jakob Disease agents can hide more virulent infections.
Manuelidis L, Yun Lu Z. Manuelidis L, et al. Neurosci Lett. 2000 Nov 3;293(3):163-6. doi: 10.1016/s0304-3940(00)01514-7. Neurosci Lett. 2000. PMID: 11036186 - Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures.
Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. Arjona A, et al. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8768-73. doi: 10.1073/pnas.0400158101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161970 Free PMC article. - Virus-like interference in the latency and prevention of Creutzfeldt-Jakob disease.
Manuelidis L, Lu ZY. Manuelidis L, et al. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5360-5. doi: 10.1073/pnas.0931192100. Epub 2003 Apr 11. Proc Natl Acad Sci U S A. 2003. PMID: 12692308 Free PMC article. - Creutzfeldt-Jakob disease and scrapie prions.
Prusiner SB. Prusiner SB. Alzheimer Dis Assoc Disord. 1989 Spring-Summer;3(1-2):52-78. doi: 10.1097/00002093-198903010-00007. Alzheimer Dis Assoc Disord. 1989. PMID: 2568118 Review.
Cited by
- Neuroinvasion by a Creutzfeldt-Jakob disease agent in the absence of B cells and follicular dendritic cells.
Shlomchik MJ, Radebold K, Duclos N, Manuelidis L. Shlomchik MJ, et al. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9289-94. doi: 10.1073/pnas.161055198. Epub 2001 Jul 24. Proc Natl Acad Sci U S A. 2001. PMID: 11470899 Free PMC article. - High titers of mucosal and systemic anti-PrP antibodies abrogate oral prion infection in mucosal-vaccinated mice.
Goñi F, Prelli F, Schreiber F, Scholtzova H, Chung E, Kascsak R, Brown DR, Sigurdsson EM, Chabalgoity JA, Wisniewski T. Goñi F, et al. Neuroscience. 2008 May 15;153(3):679-86. doi: 10.1016/j.neuroscience.2008.02.051. Epub 2008 Mar 6. Neuroscience. 2008. PMID: 18407424 Free PMC article. - High CJD infectivity remains after prion protein is destroyed.
Miyazawa K, Emmerling K, Manuelidis L. Miyazawa K, et al. J Cell Biochem. 2011 Dec;112(12):3630-7. doi: 10.1002/jcb.23286. J Cell Biochem. 2011. PMID: 21793041 Free PMC article. - Prion interference with multiple prion isolates.
Schutt CR, Bartz JC. Schutt CR, et al. Prion. 2008 Apr-Jun;2(2):61-3. doi: 10.4161/pri.2.2.6806. Epub 2008 Apr 18. Prion. 2008. PMID: 19098442 Free PMC article. Review.
References
- Halsband R. J Hist Med Allied Sci. 1953;8:390–405. - PubMed
- Sabin A B. J Biol Stand. 1973;1:115.
- Dickinson A, Fraser H, Meikle V, Outram G. Nat New Biol. 1972;237:244–245. - PubMed
- Dickinson A G, Fraser H, McConnell I, Outram G W, Sales D I, Taylor D M. Nature (London) 1975;253:556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials