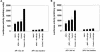The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes - PubMed (original) (raw)
The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes
K L Auer et al. Mol Biol Cell. 1998 Mar.
Free PMC article
Abstract
The ability of signaling via the JNK (c-Jun NH2-terminal kinase)/stress-activated protein kinase cascade to stimulate or inhibit DNA synthesis in primary cultures of adult rat hepatocytes was examined. Treatment of hepatocytes with media containing hyperosmotic glucose (75 mM final), tumor necrosis factor alpha (TNFalpha, 1 ng/ml final), and hepatocyte growth factor (HGF, 1 ng/ml final) caused activation of JNK1. Glucose, TNFalpha, or HGF treatments increased phosphorylation of c-Jun at serine 63 in the transactivation domain and stimulated hepatocyte DNA synthesis. Infection of hepatocytes with poly-L-lysine-coated adenoviruses coupled to constructs to express either dominant negatives Ras N17, Rac1 (N17), Cdc42 (N17), SEK1-, or JNK1- blunted the abilities of glucose, TNFalpha, or HGF to increase JNK1 activity, to increase phosphorylation of c-Jun at serine 63, and to stimulate DNA synthesis. Furthermore, infection of hepatocytes by a recombinant adenovirus expressing a dominant-negative c-Jun mutant (TAM67) also blunted the abilities of glucose, TNFalpha, and HGF to stimulate DNA synthesis. These data demonstrate that multiple agonists stimulate DNA synthesis in primary cultures of hepatocytes via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun pathway. Glucose and HGF treatments reduced glycogen synthase kinase 3 (GSK3) activity and increased c-Jun DNA binding. Co-infection of hepatocytes with recombinant adenoviruses to express dominant- negative forms of PI3 kinase (p110alpha/p110gamma) increased basal GSK3 activity, blocked the abilities of glucose and HGF treatments to inhibit GSK3 activity, and reduced basal c-Jun DNA binding. However, expression of dominant-negative PI3 kinase (p110alpha/p110gamma) neither significantly blunted the abilities of glucose and HGF treatments to increase c-Jun DNA binding, nor inhibited the ability of these agonists to stimulate DNA synthesis. These data suggest that signaling by the JNK/stress-activated protein kinase cascade, rather than by the PI3 kinase cascade, plays the pivotal role in the ability of agonists to stimulate DNA synthesis in primary cultures of rat hepatocytes.
Figures
Figure 1
(A) Hyperosmotic glucose, TNFα, or HGF treatments activate JNK1. (B) Hyperosmotic glucose, TNFα, or HGF treatments activate p70S6 kinase. (C) Hyperosmotic glucose, TNFα, or HGF treatments inhibit GSK3. (D) Modulation of p42MAP kinase activity after hyperosmotic glucose, TNFα, or HGF treatments. Hepatocytes were cultured as described in MATERIALS AND METHODS. After 4 h, cells were treated for the indicated times with either media control, 50 mM glucose (closed circles), 1 ng/μl TNFα (open triangles), or 1 ng/μl HGF (closed squares) at 37°C, after which time media were aspirated and the cells frozen. Hepatocytes were lysed and subjected to immunoprecipitation followed by immune complex kinase assays for the indicated protein kinase. Activities shown are expressed as cpm incorporated into substrate versus media control treatment (5000 cpm/pmol) and are the means of triplicates from a representative of four experiments. Media control treatment did not alter the basal activities of protein kinases versus no treatment.
Figure 2
Hyperosmotic glucose (top panel), HGF (middle panel), and TNFα (bottom panel) stimulate phosphorylation of serine 63 in the transactivation domain of c-Jun, which is blocked by expression of dominant-negative RasN17, dominant-negative RacN17, dominant-negative Cdc42N17, dominant-negative SEK1, and dominant-negative JNK1. Hepatocytes were infected with either control plasmid poly-
l
-lysine adenovirus or dominant-negative Ras N17/Rac1 N17/Cdc42 N17/SEK1/JNK1 poly-
l
-lysine adenovirus (250 moi), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative gene products, hepatocytes were treated for 20 min with either media control, 50 mM glucose, 1 ng/μl TNFα, or 1 ng/μl HGF. After 20 min, media were aspirated and cells frozen. Hepatocytes were lysed with 100 μl of 5× SDS-polyacrylamide sample buffer, diluted to 250 μl with distilled water, and placed in a 100°C dry bath for 15 min. One hundred-microliter aliquots of each time point were subjected to SDS-PAGE on two 10% gels. Gels were transferred to nitrocellulose and Western blotting versus either anti-serine 63 c-Jun or anti–c-Jun protein (performed as in MATERIALS AND METHODS). The top panels show blotting versus serine 63 c-Jun; the bottom panels show total c-Jun protein immunoblotting.
Figure 3
Hyperosmotic glucose transactivates c-Jun and stimulates luciferase activity from an AP-1-luciferase reporter construct, which is inhibited by expression of either dominant-negative SEK1 (A) or dominant-negative JNK1 (B). Hepatocytes were infected with either an AP-1-luciferase plasmid poly-
l
-lysine adenovirus or a mutant AP-1-luciferase poly-
l
-lysine adenovirus (100 moi) and coinfected in combination with either control plasmid poly-
l
-lysine adenovirus, dominant-negative SEK1 plasmid poly-
l
-lysine adenovirus, or dominant- negative JNK1 plasmid poly-
l
-lysine adenovirus (250 moi), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative SEK1 or dominant-negative JNK1, hepatocytes were treated for another 12 h with either media control or 50 mM glucose, after which media were aspirated and the cells frozen. Cells were prepared for assay of luciferase activity using a kit, as described in the manufacturer’s instructions [see MATERIALS AND METHODS (de Wet et al., 1987)]. Data are means ± SE from the averages of triplicates (six data points) from two experiments.
Figure 4
Coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ block the ability of hyperosmotic glucose treatment and HGF treatment to activate p70S6 kinase and to inhibit GSK3 activities. Hepatocytes were infected with either control recombinant adenovirus (250 moi) or recombinant adenoviruses to express dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ (125 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ, hepatocytes were treated for 10 min at 37°C with either media control, 50 mM glucose, or 1 ng/μl HGF. After 10 min, media were aspirated and the cells frozen. Hepatocytes were lysed and subjected to immunoprecipitation, followed by immune complex kinase assays for both GSK3 and p70S6 kinase. Activities shown are expressed as cpm incorporated into substrate versus media control treatment (5000 cpm/pmol) and are the means ± SE of duplicates from four experiments. Media control treatment did not alter the basal activities of protein kinases versus no treatment.
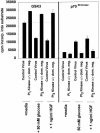
Figure 5
Hyperosmotic glucose (A) and HGF (B) treatments stimulate DNA binding of AP-1/c-Jun complex, which is not blocked by coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ. Hepatocytes were infected with either control recombinant adenovirus (250 moi) or recombinant adenoviruses to express dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ (125 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ, hepatocytes were treated for 20 min at 37°C with either media control, 50 mM glucose, or 1 ng/μl HGF. After 20 min, media were aspirated and the cells frozen. Hepatocytes were lysed, nuclear extracts prepared, and AP-1 DNA-binding experiments performed as in MATERIALS AND METHODS. Data are representative of four independent experiments. No alteration in c-Jun/AP-1 DNA binding was observed in media control samples. Lane 1, control virus + 50 mM glucose; lane 2, control virus; lane 3, dominant-negative PI3 kinase (p110α and p110γ) + 50 mM glucose; and lane 4, PI3 kinase (p110α and p110γ).
Similar articles
- The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic.
Tombes RM, Auer KL, Mikkelsen R, Valerie K, Wymann MP, Marshall CJ, McMahon M, Dent P. Tombes RM, et al. Biochem J. 1998 Mar 15;330 ( Pt 3)(Pt 3):1451-60. doi: 10.1042/bj3301451. Biochem J. 1998. PMID: 9494119 Free PMC article. - Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade.
Hu MC, Qiu WR, Wang X, Meyer CF, Tan TH. Hu MC, et al. Genes Dev. 1996 Sep 15;10(18):2251-64. doi: 10.1101/gad.10.18.2251. Genes Dev. 1996. PMID: 8824585 - Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades.
Lopez-Ilasaca M. Lopez-Ilasaca M. Biochem Pharmacol. 1998 Aug 1;56(3):269-77. doi: 10.1016/s0006-2952(98)00059-8. Biochem Pharmacol. 1998. PMID: 9744561 Review. - Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists.
Clerk A, Sugden PH. Clerk A, et al. Am J Cardiol. 1999 Jun 17;83(12A):64H-69H. doi: 10.1016/s0002-9149(99)00261-1. Am J Cardiol. 1999. PMID: 10750590 Review.
Cited by
- Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines.
Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, Zhang L, Huang H, Zhang D, Wu XR, Gao J, Huang C. Jin H, et al. Oncotarget. 2015 Jan 1;6(1):522-36. doi: 10.18632/oncotarget.2680. Oncotarget. 2015. PMID: 25402510 Free PMC article. - IGFBP-3 inhibits TNF-α production and TNFR-2 signaling to protect against retinal endothelial cell apoptosis.
Zhang Q, Steinle JJ. Zhang Q, et al. Microvasc Res. 2014 Sep;95:76-81. doi: 10.1016/j.mvr.2014.07.009. Epub 2014 Jul 30. Microvasc Res. 2014. PMID: 25086184 Free PMC article. - P2Y2 nucleotide receptor activation enhances the aggregation and self-organization of dispersed salivary epithelial cells.
El-Sayed FG, Camden JM, Woods LT, Khalafalla MG, Petris MJ, Erb L, Weisman GA. El-Sayed FG, et al. Am J Physiol Cell Physiol. 2014 Jul 1;307(1):C83-96. doi: 10.1152/ajpcell.00380.2013. Epub 2014 Apr 23. Am J Physiol Cell Physiol. 2014. PMID: 24760984 Free PMC article. - Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention.
Ip BC, Wang XD. Ip BC, et al. Nutrients. 2013 Dec 27;6(1):124-62. doi: 10.3390/nu6010124. Nutrients. 2013. PMID: 24379011 Free PMC article. Review. - Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway in human hepatoma SMMC-7721 cells.
Zhang Z, Miao L, Lv C, Sun H, Wei S, Wang B, Huang C, Jiao B. Zhang Z, et al. Cell Death Dis. 2013 Jun 6;4(6):e657. doi: 10.1038/cddis.2013.182. Cell Death Dis. 2013. PMID: 23744357 Free PMC article.
References
- Bagrodia S, Derijard B, Davis RJ, Cerione R. Cdc42 and PAK-mediated signaling leads to JNK and p38 MAP kinase activation. J Biol Chem. 1995;270:27995–27998. - PubMed
- Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated protein kinase subfamilies in perfused heart. Circ Res. 1996;79:162–173. - PubMed
- Boylan JM, Gruppuso PA. A comparative study of the hepatic mitogen activated protein kinase and JNK pathways in late gestational fetal rat. Cell Growth & Differ. 1996;7:1261–1269. - PubMed
- Boyle WJ, Smeal T, Defize LHK, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA binding ability. Cell. 1990;64:573–584. - PubMed
- Bruccderi A, Gallucci R, Germolec DR, Blackshear P, Simeonova P, Thurman RG, Luster MI. Induction of early-immediate genes by TNFα contribute to liver repair following chemical induced hepatotoxicity. Hepatology. 1997;25:133–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous