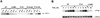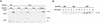Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line - PubMed (original) (raw)
Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line
B L Williams et al. Mol Cell Biol. 1998 Mar.
Abstract
T-cell antigen receptor (TCR) engagement activates multiple protein tyrosine kinases (PTKs), including the Src family member, Lck, and the Syk-related PTK, ZAP-70. Studies in ZAP-70-deficient humans have demonstrated that ZAP-70 plays crucial roles in T-cell activation and development. However, progress toward a detailed understanding of the regulation and function of ZAP-70 during TCR signaling has been hampered by the lack of a suitable T-cell model for biochemical and genetic analyses. In this report, we describe the isolation and phenotypic characterization of a Syk- and ZAP-70-negative somatic mutant derived from the Jurkat T-cell line. The P116 cell line displays severe defects in TCR-induced signaling functions, including protein tyrosine phosphorylation, intracellular Ca2+ mobilization, and interleukin-2 promoter-driven transcription. These signaling defects were fully reversed by reintroduction of catalytically active versions of either Syk or ZAP-70 into the P116 cells. However, in contrast to ZAP-70 expression, Syk expression triggered a significant degree of cellular activation in the absence of TCR ligation. Transfection experiments with ZAP-70-Syk chimeric proteins indicated that both the amino-terminal regulatory regions and the carboxy-terminal catalytic domains of Syk and ZAP-70 contribute to the distinctive functional properties of these PTKs. These studies underscore the crucial role of ZAP-70 in TCR signaling and offer a powerful genetic model for further analyses of ZAP-70 regulation and function in T cells.
Figures
FIG. 1
Stimulus-induced calcium mobilization in wild-type and mutant Jurkat cell lines. Jurkat subclones were loaded with indo-1 and stimulated with 1 μg of anti-CD3 antibody (OKT3) per ml or 0.1 mM pervanadate (PV). The ratio of the fluorescence emission of the Ca2+-bound to -free form of indo-1 is plotted as a function of time after addition of the stimulus. The cells tested in each panel are as follows: JURKAT, wild-type Jurkat cells; J.CaM1, Lck-negative Jurkat subclone; P116, ZAP-70-negative Jurkat subclone; P116.C39 and P116.C40, ZAP-70-transfected P116 subclones; DK33, P116 subclone transfected with a catalytically inactive ZAP-70 mutant.
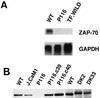
FIG. 2
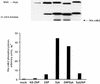
ZAP-70 expression in Jurkat-derived subclones. (A) Northern blot analysis. Total cellular RNA (30 μg) from Jurkat cells (wild type [WT]), P116 cells, or TF.wild B-lymphoma cells was separated electrophoretically and blotted onto a nitrocellulose membrane. The Northern blot was sequentially hybridized with 32P-labeled DNA probes for ZAP-70 and GAPDH. (B) Immunoblot analysis. Detergent-soluble proteins from wild-type Jurkat or the indicated Jurkat subclones (see Fig. 1 legend for description of subclones) were separated by SDS-PAGE and immunoblotted with ZAP-70-specific antibodies. The decreased electrophoretic mobility of the kinase-inactive ZAP-70 mutant expressed in DK2 and DK33 cells is due to the presence of the amino-terminal Myc epitope tag.
FIG. 3
Stimulus-induced protein tyrosine phosphorylation in Jurkat-derived subclones. Wild-type (WT) Jurkat cells or Jurkat-derived subclones were stimulated for 2 min with medium only (CTRL), 1 μg of anti-CD3 (OKT3) antibody per ml cross-linked with 10 μg of RaMIg per ml, or 0.1 mM pervanadate (PV). Detergent-soluble proteins were separated by SDS-PAGE, followed by immunoblotting with antiphosphotyrosine MAb. The numbers on the left indicate molecular mass in kilodaltons.
FIG. 4
PLC-γ1 phosphorylation in Jurkat-derived subclones. (A) Phosphorylation of PLC-γ1 in Jurkat subclones. Wild-type (WT) Jurkat cells and the indicated Jurkat subclones were stimulated for 2 min with either anti-CD3 antibody (OKT3) or pervanadate (PV), and cellular extracts were immunoprecipitated with anti-PLC-γ1 antibodies. The immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted with antiphosphotyrosine MAb. (B) Time course of pervanadate-induced tyrosine phosphorylation of PLC-γ1. The indicated Jurkat cell lines were stimulated for the various times with PV. The cells were lysed, and PLC-γ1 immunoprecipitates were immunoblotted with antiphosphotyrosine antibodies as described in panel A.
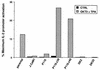
FIG. 5
IL-2 promoter-driven transcription in Jurkat subclones. Wild-type Jurkat cells or the indicated Jurkat subclones were transiently transfected with 10 μg of pIL2-Luc reporter plasmid. After 12 h, transfected cell populations were divided into three equivalent samples. The samples were stimulated for 12 h with medium only, 1 μg of anti-CD3 antibodies (OKT3) per ml plus 20 ng of TPA per ml or 2 μM ionomycin plus 20 ng of TPA per ml. Luciferase activities were plotted as a percentage of the maximal activity induced by ionomycin plus TPA in each cell population. The results shown are representative of seven independent trials. The P116.c39 and P116.c40 cell lines are stable transfectants expressing wild-type ZAP-70, and the DK2 and DK33 cell lines are stably transfected with a catalytically inactive ZAP-70 mutant.
FIG. 6
Whole-cell protein tyrosine phosphorylation in Jurkat subclones infected with Syk- or ZAP-encoding vaccinia virus. Wild-type (WT) Jurkat, J.CaM1, or P116 cells were infected with control vaccinia virus strain WR or with recombinant vaccinia virus encoding ZAP-70 or Syk. Three hours after infection, cells were stimulated for 1 min with medium only or with anti-CD3 antibody cross-linked with goat anti-mouse IgG. Detergent-soluble proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine MAb.
FIG. 7
TCR ζ subunit phosphorylation in P116 cells expressing ZAP-70 or Syk. (A) Cells were infected with nonrecombinant (WR) or recombinant vaccinia viruses and stimulated with OKT3 as described in the legend for Fig. 6. The samples labeled KD-ZAP were from cells infected with vaccinia virus encoding a catalytically inactive ZAP-70 mutant (Lys349 → Arg). At 3 h postinfection, the cells were stimulated for 1 min with medium only or with OKT3 cross-linked with goat anti-mouse IgG. Detergent-soluble proteins were immunoprecipitated with ζ-specific antibodies. The immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted with antiphosphotyrosine (upper panel) and then stripped and reblotted with anti-ζ antibodies (lower panel). (B) Time course of ζ subunit phosphorylation in ZAP-70 versus Syk-expressing P116 cells.
FIG. 8
Reconstitution of gene transcription in P116 cells expressing ZAP-70 or Syk. (A) Effect of Syk versus ZAP-70 on NFAT-driven gene transcription. P116 cells were transfected with 10 μg of pNFAT-Luc in the presence of empty vector (mock) or the indicated amounts of pcDNA3-mZAP or pcDNA3-mSyk. After transfection, equivalent samples of each cell population were stimulated for 6 h with medium only (unstim) or with 1 μg of anti-CD3 antibody (OKT3) per ml. Cellular extracts were assayed for luciferase activity and for expression of epitope-tagged proteins by immunoblotting with 9E10 MAb. The results shown are representative of four separate trials. (B) TCR-dependent effects of Syk and ZAP-70 on NFAT-driven gene transcription. P116 and TCR-negative J.RT3 cells were transfected with 10 μg of pNFAT-Luc and 10 μg of empty vector or the indicated plasmid. Samples were processed as in panel A. The results shown are representative of three independent experiments.
FIG. 9
Effect of ZAP-70–Syk chimeric proteins on NFAT-dependent transcription. (A) Structures of ZAP-70–Syk chimeric proteins. The numbers below each construct indicate amino acid residue numbers from the full-length ZAP-70 and Syk proteins. (B) Reconstitution of TCR signaling functions with ZAP-70–Syk chimeric proteins. P116 cells were transfected with pNFAT-Luc along with 10 μg of either empty vector (mock), pcDNA3-mZAP, pcDNA3-mSyk, or the indicated amounts of pcDNA3-mZAPSyk or pcDNA3-mSykZAP. The cells were stimulated, and luciferase assays were done as described in Fig. 8A. (C) Effects of ZAP-70–Syk chimeric PTKs on SLP-76 tyrosine phosphorylation. P116 cells were stably transfected with wild-type ZAP-70 (P116.c39) or with Syk-ZAP (clones SZ-10 and -13) or ZAP-Syk (clones ZS-12 and -58) expression vectors. The indicated clones were selected based on expression of the transfected PTK at levels approximating those found in the parental Jurkat cell line (62). The cells were stimulated for 2 min with anti-CD3 antibodies (OKT3), and detergent-soluble proteins were immunoprecipitated with anti-SLP-76 antibodies. After separation by SDS-PAGE, the immunoprecipitated proteins were immunoblotted with antiphosphotyrosine antibodies (upper panel). The blot was stripped and reprobed with anti-SLP-76 antibodies (lower panel). The apparent decrease in band intensity in stimulated sample lanes is due to the reduced immunoreactivity of the antibodies with the tyrosine-phosphorylated forms of SLP-76 (62). (D) Role of Lck in NFAT activation by ZAP-70–Syk chimeric proteins. J.CaM1 cells were transfected with 10 μg of pNFAT-Luc along with 40 μg of empty vector (mock) or with the indicated expression plasmid. Stimulations and luciferase assays were performed as described in the legend for Fig. 8. The results in panels B and D are each representative of three separate trials.
FIG. 9
Effect of ZAP-70–Syk chimeric proteins on NFAT-dependent transcription. (A) Structures of ZAP-70–Syk chimeric proteins. The numbers below each construct indicate amino acid residue numbers from the full-length ZAP-70 and Syk proteins. (B) Reconstitution of TCR signaling functions with ZAP-70–Syk chimeric proteins. P116 cells were transfected with pNFAT-Luc along with 10 μg of either empty vector (mock), pcDNA3-mZAP, pcDNA3-mSyk, or the indicated amounts of pcDNA3-mZAPSyk or pcDNA3-mSykZAP. The cells were stimulated, and luciferase assays were done as described in Fig. 8A. (C) Effects of ZAP-70–Syk chimeric PTKs on SLP-76 tyrosine phosphorylation. P116 cells were stably transfected with wild-type ZAP-70 (P116.c39) or with Syk-ZAP (clones SZ-10 and -13) or ZAP-Syk (clones ZS-12 and -58) expression vectors. The indicated clones were selected based on expression of the transfected PTK at levels approximating those found in the parental Jurkat cell line (62). The cells were stimulated for 2 min with anti-CD3 antibodies (OKT3), and detergent-soluble proteins were immunoprecipitated with anti-SLP-76 antibodies. After separation by SDS-PAGE, the immunoprecipitated proteins were immunoblotted with antiphosphotyrosine antibodies (upper panel). The blot was stripped and reprobed with anti-SLP-76 antibodies (lower panel). The apparent decrease in band intensity in stimulated sample lanes is due to the reduced immunoreactivity of the antibodies with the tyrosine-phosphorylated forms of SLP-76 (62). (D) Role of Lck in NFAT activation by ZAP-70–Syk chimeric proteins. J.CaM1 cells were transfected with 10 μg of pNFAT-Luc along with 40 μg of empty vector (mock) or with the indicated expression plasmid. Stimulations and luciferase assays were performed as described in the legend for Fig. 8. The results in panels B and D are each representative of three separate trials.
FIG. 9
Effect of ZAP-70–Syk chimeric proteins on NFAT-dependent transcription. (A) Structures of ZAP-70–Syk chimeric proteins. The numbers below each construct indicate amino acid residue numbers from the full-length ZAP-70 and Syk proteins. (B) Reconstitution of TCR signaling functions with ZAP-70–Syk chimeric proteins. P116 cells were transfected with pNFAT-Luc along with 10 μg of either empty vector (mock), pcDNA3-mZAP, pcDNA3-mSyk, or the indicated amounts of pcDNA3-mZAPSyk or pcDNA3-mSykZAP. The cells were stimulated, and luciferase assays were done as described in Fig. 8A. (C) Effects of ZAP-70–Syk chimeric PTKs on SLP-76 tyrosine phosphorylation. P116 cells were stably transfected with wild-type ZAP-70 (P116.c39) or with Syk-ZAP (clones SZ-10 and -13) or ZAP-Syk (clones ZS-12 and -58) expression vectors. The indicated clones were selected based on expression of the transfected PTK at levels approximating those found in the parental Jurkat cell line (62). The cells were stimulated for 2 min with anti-CD3 antibodies (OKT3), and detergent-soluble proteins were immunoprecipitated with anti-SLP-76 antibodies. After separation by SDS-PAGE, the immunoprecipitated proteins were immunoblotted with antiphosphotyrosine antibodies (upper panel). The blot was stripped and reprobed with anti-SLP-76 antibodies (lower panel). The apparent decrease in band intensity in stimulated sample lanes is due to the reduced immunoreactivity of the antibodies with the tyrosine-phosphorylated forms of SLP-76 (62). (D) Role of Lck in NFAT activation by ZAP-70–Syk chimeric proteins. J.CaM1 cells were transfected with 10 μg of pNFAT-Luc along with 40 μg of empty vector (mock) or with the indicated expression plasmid. Stimulations and luciferase assays were performed as described in the legend for Fig. 8. The results in panels B and D are each representative of three separate trials.
FIG. 9
Effect of ZAP-70–Syk chimeric proteins on NFAT-dependent transcription. (A) Structures of ZAP-70–Syk chimeric proteins. The numbers below each construct indicate amino acid residue numbers from the full-length ZAP-70 and Syk proteins. (B) Reconstitution of TCR signaling functions with ZAP-70–Syk chimeric proteins. P116 cells were transfected with pNFAT-Luc along with 10 μg of either empty vector (mock), pcDNA3-mZAP, pcDNA3-mSyk, or the indicated amounts of pcDNA3-mZAPSyk or pcDNA3-mSykZAP. The cells were stimulated, and luciferase assays were done as described in Fig. 8A. (C) Effects of ZAP-70–Syk chimeric PTKs on SLP-76 tyrosine phosphorylation. P116 cells were stably transfected with wild-type ZAP-70 (P116.c39) or with Syk-ZAP (clones SZ-10 and -13) or ZAP-Syk (clones ZS-12 and -58) expression vectors. The indicated clones were selected based on expression of the transfected PTK at levels approximating those found in the parental Jurkat cell line (62). The cells were stimulated for 2 min with anti-CD3 antibodies (OKT3), and detergent-soluble proteins were immunoprecipitated with anti-SLP-76 antibodies. After separation by SDS-PAGE, the immunoprecipitated proteins were immunoblotted with antiphosphotyrosine antibodies (upper panel). The blot was stripped and reprobed with anti-SLP-76 antibodies (lower panel). The apparent decrease in band intensity in stimulated sample lanes is due to the reduced immunoreactivity of the antibodies with the tyrosine-phosphorylated forms of SLP-76 (62). (D) Role of Lck in NFAT activation by ZAP-70–Syk chimeric proteins. J.CaM1 cells were transfected with 10 μg of pNFAT-Luc along with 40 μg of empty vector (mock) or with the indicated expression plasmid. Stimulations and luciferase assays were performed as described in the legend for Fig. 8. The results in panels B and D are each representative of three separate trials.
FIG. 10
Catalytic activities of ZAP-70–Syk chimeric proteins. K562 cells were transfected with the indicated expression plasmids. Cellular extracts were immunoprecipitated with the tag-specific 9E10 MAb. Immune complex kinase reactions were done with [γ-32P]ATP and 2 μg of His-tagged cdb3 as the substrate. Radiolabeled proteins were detected by autoradiography (middle panel), and the incorporation of 32P into His-cdb3 was quantitated with an AMBIS phosphorimager (bottom panel). The immunoprecipitated protein tyrosine kinases were detected by immunoblotting with 9E10 MAb (upper panel).
Similar articles
- Genetic evidence of a role for Lck in T-cell receptor function independent or downstream of ZAP-70/Syk protein tyrosine kinases.
Wong J, Straus D, Chan AC. Wong J, et al. Mol Cell Biol. 1998 May;18(5):2855-66. doi: 10.1128/MCB.18.5.2855. Mol Cell Biol. 1998. PMID: 9566904 Free PMC article. - T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70.
Zeitlmann L, Knorr T, Knoll M, Romeo C, Sirim P, Kolanus W. Zeitlmann L, et al. J Biol Chem. 1998 Jun 19;273(25):15445-52. doi: 10.1074/jbc.273.25.15445. J Biol Chem. 1998. PMID: 9624129 - Regulation of T-cell antigen receptor signalling by Syk tyrosine protein kinase.
Latour S, Fournel M, Veillette A. Latour S, et al. Mol Cell Biol. 1997 Aug;17(8):4434-41. doi: 10.1128/MCB.17.8.4434. Mol Cell Biol. 1997. PMID: 9234701 Free PMC article. - The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes.
van Oers NS, Weiss A. van Oers NS, et al. Semin Immunol. 1995 Aug;7(4):227-36. doi: 10.1006/smim.1995.0027. Semin Immunol. 1995. PMID: 8520027 Review. - Regulating the discriminatory response to antigen by T-cell receptor.
Gangopadhyay K, Roy S, Sen Gupta S, Chandradasan AC, Chowdhury S, Das R. Gangopadhyay K, et al. Biosci Rep. 2022 Mar 31;42(3):BSR20212012. doi: 10.1042/BSR20212012. Biosci Rep. 2022. PMID: 35260878 Free PMC article. Review.
Cited by
- TNFR-associated factor 6 regulates TCR signaling via interaction with and modification of LAT adapter.
Xie JJ, Liang JQ, Diao LH, Altman A, Li Y. Xie JJ, et al. J Immunol. 2013 Apr 15;190(8):4027-36. doi: 10.4049/jimmunol.1202742. Epub 2013 Mar 20. J Immunol. 2013. PMID: 23514740 Free PMC article. - Naturally Occurring Genetic Alterations in Proximal TCR Signaling and Implications for Cancer Immunotherapy.
Kent A, Longino NV, Christians A, Davila E. Kent A, et al. Front Immunol. 2021 May 3;12:658611. doi: 10.3389/fimmu.2021.658611. eCollection 2021. Front Immunol. 2021. PMID: 34012443 Free PMC article. Review. - A Cysteine Residue within the Kinase Domain of Zap70 Regulates Lck Activity and Proximal TCR Signaling.
Schultz A, Schnurra M, El-Bizri A, Woessner NM, Hartmann S, Hartig R, Minguet S, Schraven B, Simeoni L. Schultz A, et al. Cells. 2022 Sep 1;11(17):2723. doi: 10.3390/cells11172723. Cells. 2022. PMID: 36078131 Free PMC article. - Natural isoaspartyl protein modification of ZAP70 alters T cell responses in lupus.
Yang ML, Lam TT, Kanyo J, Kang I, Zhou ZS, Clarke SG, Mamula MJ. Yang ML, et al. Autoimmunity. 2023 Dec;56(1):2282945. doi: 10.1080/08916934.2023.2282945. Epub 2023 Nov 23. Autoimmunity. 2023. PMID: 37994408 Free PMC article.
References
- Appleby M W, Gross J A, Cooke M P, Levin S D, Qian X, Perlmutter R M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. - PubMed
- Arpaia E, Shahar M, Dadi H, Cohen A, Roifman C M. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking Zap-70 kinase. Cell. 1994;76:947–958. - PubMed
- Binstadt B A, Brumbaugh K M, Dick C J, Scharenberg A M, Williams B L, Colonna M, Lanier L L, Kinet J-P, Abraham R T, Leibson P J. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous