A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway - PubMed (original) (raw)
A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway
S H Liu et al. J Cell Biol. 1998.
Abstract
The role of clathrin in intracellular sorting was investigated by expression of a dominant-negative mutant form of clathrin, termed the hub fragment. Hub inhibition of clathrin-mediated membrane transport was established by demonstrating a block of transferrin internalization and an alteration in the intracellular distribution of the cation-independent mannose-6-phosphate receptor. Hubs had no effect on uptake of FITC-dextran, adaptor distribution, organelle integrity in the secretory pathway, or cell surface expression of constitutively secreted molecules. Hub expression blocked lysosomal delivery of chimeric molecules containing either the tyrosine-based sorting signal of H2M or the dileucine-based sorting signal of CD3gamma, confirming a role for clathrin-coated vesicles (CCVs) in recognizing these signals and sorting them to the endocytic pathway. Hub expression was then used to probe the role of CCVs in targeting native molecules bearing these sorting signals in the context of HLA-DM and the invariant chain (I chain) complexed to HLA-DR. The distribution of these molecules was differentially affected. Accumulation of hubs before expression of the DM dimer blocked DM export from the TGN, whereas hubs had no effect on direct targeting of the DR-I chain complex from the TGN to the endocytic pathway. However, concurrent expression of hubs, such that hubs were building to inhibitory concentrations during DM or DR-I chain expression, caused cell surface accumulation of both complexes. These observations suggest that both DM and DR-I chain are directly transported to the endocytic pathway from the TGN, DM in CCVs, and DR-I chain independent of CCVs. Subsequently, both complexes can appear at the cell surface from where they are both internalized by CCVs. Differential packaging in CCVs in the TGN, mediated by tyrosine- and dileucine-based sorting signals, could be a mechanism for functional segregation of DM from DR-I chain until their intended rendezvous in late endocytic compartments.
Figures
Figure 1
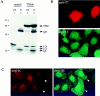
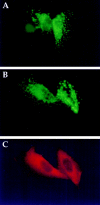
Transfected hubs alter the intracellular distribution of both clathrin heavy chains and LCs. (A) HeLa cells were transfected with control vector (pCDM8 without the T7Hub insert, lanes 1 and 2) or with T7Hub (pCDM8T7Hub vector, lanes 3 and 4). 48 h after transfection, cells were lysed and then the T7Hub protein was immunoprecipitated with anti-T7 mAb bound to protein G–Sepharose. The unbound (UB) and bound (B) fractions were then analyzed by SDS-PAGE and immunoblotting. The T7Hub protein and clathrin LCs (LCa and LCb) were detected with anti-T7 mAb and anti-LC antiserum, respectively. IgH and IgL are the subunits of the mAb used for immunoprecipitation, detected by the secondary anti-immunoglobulin antibody. (B) HeLa cells were transfected with T7Hub. 48 h after transfection, cells were processed for immunofluorescent microscopy and stained with anti-T7 mAb and anti-LC antiserum (anti-LC) followed by LRSC-conjugated goat anti–mouse IgG and FITC-conjugated goat anti–rabbit IgG. (C) T7Hub-transfected HeLa cells were permeabilized with 0.004% digitonin to remove cytosol before fixation and thereby visualize membrane-associated clathrin. The cells were stained with anti-LC antiserum (anti-LC) and antiheavy chain mAb X32 (anti-HC) followed by rhodamine- conjugated goat anti–rabbit IgG and FITC-conjugated goat anti–mouse IgG. The T7Hub-transfected cells were identified by the loss of punctate clathrin LC staining (arrowheads) and are the ones with increased staining of clathrin heavy chain at the PM.
Figure 2
Expression of T7Hub in transfected cells does not alter the general distribution of adaptor molecules. HeLa cells were transfected with T7Hub and after 48 h were then analyzed by immunofluorescence for the distribution of the AP1 and AP2 adaptors. (A) Cells stained with anti-AP2 mAb AP.6 (AP2) and biotinylated anti-T7 mAb followed by FITC-conjugated anti–mouse IgG1–specific secondary antibody (for AP.6) and TRITC-conjugated streptavidin. (B) Cells stained with anti-AP1 mAb 100/3 (AP1) and anti-LC (anti-LC) antiserum followed by LRSC-conjugated goat anti–mouse IgG and FITC-conjugated goat anti– rabbit IgG. The T7Hub– transfected cells in B were identified by the loss of punctate clathrin LC staining (arrowheads).
Figure 3
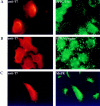
Expression of T7Hub in transfected cells inhibits receptor-mediated endocytosis but not bulk fluid-phase uptake and alters the intracellular distribution of the CI-M6PR. (A) HeLa cells were transfected with T7Hub. 48 h after transfection, cells were allowed to internalize FITC-Tfn for 10 min at 37°C, and then were processed for immunofluorescent microscopy. Hub-transfected cells were visualized by anti-T7 mAb and LRSC-conjugated goat anti–mouse IgG. (B) HeLa cells were transfected with T7Hub and, after 48 h, were allowed to internalize FITC-dextran at 37°C for 3 h before being processed for anti-T7 mAb staining to visualize cells with hubs. (C) HeLa cells were transfected with T7Hub and after 48 h were processed for immunofluorescent microscopy to visualize the CI-M6PR, with specific rabbit antiserum and FITC-conjugated goat anti–rabbit IgG, and hub-transfected cells, with anti-T7 mAb and LRSC-conjugated goat anti–mouse IgG.
Figure 4
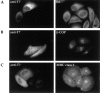
Hub expression does not have pleiotropic effects on organelle integrity or the constitutive secretory pathway. HeLa cells were transfected with T7Hub and, after 48 h, were stained with (A) anti-ER antiserum and FITC-conjugated goat anti– rabbit IgG, (B) anti–β-COP mAb M3A5 and FITC-conjugated goat anti–mouse IgG1–specific secondary antibody, or (C) anti-MHC class I mAb W6/32 and FITC-conjugated anti–mouse IgG2a–specific secondary antibody. The T7Hub-transfected cells in each field were visualized either by (A) anti-T7 mAb followed by LRSC-conjugated goat anti–mouse IgG or (B and C) biotinylated anti-T7 mAb followed by TRITC-conjugated streptavidin.
Figure 5
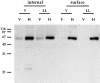
Expression of T7Hub increases steady-state levels of cotransfected Tac chimeras bearing tyrosine- and dileucine-based sorting motifs and causes their cell surface accumulation. HeLa cells were transfected with control vector without the hub insert (V) or T7Hub (H). Each population of cells was cotransfected with vector encoding the Tac chimera with the tyrosine-based sorting signal of H2M (Y) or vector encoding the Tac chimera with the dileucine-based sorting motif of CD3γ (LL). 48 h after transfection, cells were surface biotinylated and lysates were prepared. Surface molecules were removed from the lysate with avidin–agarose and then internal molecules were left in the unbound fraction. The presence of Tac chimeras in each fraction of the lysate was established by immunoblotting with anti-Tac antiserum after SDS-PAGE. For each sample, 100% of the surface fraction and 25% of the internal fraction were analyzed.
Figure 6
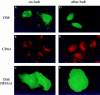
Expression of T7Hub results in cellular redistribution of cotransfected Tac chimeras bearing tyrosine- and dileucine-based sorting motifs. HeLa cells were transfected with (A and D) control vector (−hub) or (B, C, E, and F) T7Hub (+hub) together with (A–C) vector encoding the Tac chimera with a tyrosine-based sorting signal (Y motif) or (D–F) vector encoding the Tac chimera with a dileucine-based sorting signal (LL motif). 48 h after transfection, cells were processed for immunofluorescence and stained with (A, B, D, and E) anti-Tac antiserum followed by FITC-conjugated goat anti–rabbit IgG and (C and F) anti-T7 mAb followed by LRSC-conjugated goat anti–mouse IgG.
Figure 7
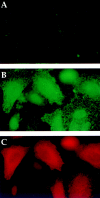
Hub expression results in surface accumulation of coexpressed HLA–DM molecules. HeLa cells were transfected with either (A) control vector (no hub) or (B and C) T7Hub and (A–C) cotransfected with vectors encoding the HLA–DM α/β dimer (pCDM8DMα and pCDM8DMβ). (A and B) Surface expression of DM was analyzed by incubating nonpermeabilized, live cells with anti-DM antiserum for 30 min on ice. Cells were then fixed and processed for immunofluorescent microscopy using FITC-conjugated goat anti–rabbit IgG to detect surface DM. (C) Cells in B were labeled with anti-T7 mAb followed by LRSC-conjugated goat anti–mouse IgG to identify cells with hubs.
Figure 8
Direct delivery of HLA–DM molecules from the TGN to lysosome-like compartments is inhibited by hubs. HeLa cells were transfected with (A–C) control vector or (D–F) T7Hub. (A, B, D, and E) were cotransfected with vectors encoding the HLA–DM α/β dimer driven by the inducible human metallothionein promoter (pMTΔ302DMα and pMTΔ302DMβ). C and F were cotransfected with a vector encoding the DMβ chain without the YTPL internalization motif (pCDM8.1.DMβ-HSSΔ) and the inducible DMα chain (pMTΔ302DMα). 24 h after transfection, expression of DM molecules was induced by exposure to 10 μM CdCl2 for 20 h. Cells were fixed, permeabilized, and then processed for intracellular immunofluorescence. Colocalization of DM relative to the lysosomal marker CD63 was analyzed by double-staining cells with (A and D) anti-DM antiserum followed by FITC-conjugated goat anti–rabbit IgG and (B and E) anti-CD63 mAb followed by LRSC-conjugated goat anti–mouse IgG. Note that in A and D, DM is detected only in transfected cells that are cotransfected with control vector or hub, whereas CD63 (B and E) is detected in all cells. The surface staining of DM in comparable samples was barely detectable for both control and hub-transfected cells (data not shown). In C and F the surface expression of the DMβ chain without the YTPL internalization motif occured when expression of cotransfected DMα was induced and was detected in control or hub-transfected cells by labeling with anti-DM antiserum followed by FITC-conjugated goat anti– rabbit IgG.
Figure 9
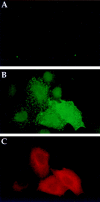
Hub expression results in the surface accumulation of coexpressed I chain. HeLa cells were transfected with (A) control vector (no hub) or (B and C) T7Hub and (A, B, and C) cotransfected with vectors encoding I chain and HLA–DRα and β chains (pCDM8Ip33, pCDM8DRα, pCDM8DRβ). (A and B) Surface expression of I chain was analyzed by incubating nonpermeabilized, live cells with anti-I chain antiserum KESL for 30 min on ice. Cells were then fixed and processed for immunofluorescent microscopy using FITC-conjugated goat anti–rabbit IgG to detect surface I chain. (C) Cells in B were labeled with anti-T7 mAb followed by LRSC-conjugated goat anti–mouse IgG to identify cells with hubs.
Figure 10
Direct delivery of I chain (coexpressed with DR) from the TGN to the endocytic pathway is not affected by expression of hubs. HeLa cells were transfected with (A) control vector (no hub) or (B and C) T7Hub and (A–C) cotransfected with vectors encoding HLA-DR α and β chains (pCDM8DRα and pCDM8DRβ) and the I chain driven by human metallothionein promoter (pMTΔ302Ip33). 24 h after transfection, I chain expression was induced by exposure to 10 μM CdCl2 for 20 h. Cells were fixed, permeabilized, and then processed for immunofluorescence. The cells were stained with (A and B) anti-I chain mAb PIN.1.1, followed by FITC-conjugated goat anti–mouse IgG1– specific secondary antibody and then (C) biotinylated anti-T7 mAb followed by TRITC-conjugated streptavidin.
Figure 11
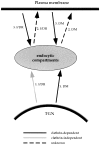
The role of clathrin and routes of transport in targeting I chain–class II MHC complexes and HLA–DM to the endocytic pathway. Analysis of DM and I chain–class II targeting, in the presence of hub molecules that inhibit CCV–mediated transport, suggests that both DM and I chain–DR are directly transported to the endocytic pathway from the TGN, without initial routing via the PM. In the TGN, DM is sorted in CCV and I chain–DR by a clathrin-independent mechanism. Our results also indicate that both complexes can subsequently appear on the cell surface and are then internalized by CCV, hence we propose they can follow routes 2 and 3, as shown. Solid arrows indicate pathways determined by the data reported, with clathrin-dependence shown in black and clathrin-independent pathways depicted in gray. Dashed arrows indicate speculated pathways where the role of clathrin is unknown. The detailed rationale behind this transport scheme is presented in the Discussion.
Similar articles
- The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles.
Höning S, Griffith J, Geuze HJ, Hunziker W. Höning S, et al. EMBO J. 1996 Oct 1;15(19):5230-9. EMBO J. 1996. PMID: 8895568 Free PMC article. - Endocytic clathrin-coated pit formation is independent of receptor internalization signal levels.
Santini F, Marks MS, Keen JH. Santini F, et al. Mol Biol Cell. 1998 May;9(5):1177-94. doi: 10.1091/mbc.9.5.1177. Mol Biol Cell. 1998. PMID: 9571248 Free PMC article. - The biogenesis of the MHC class II compartment in human I-cell disease B lymphoblasts.
Glickman JN, Morton PA, Slot JW, Kornfeld S, Geuze HJ. Glickman JN, et al. J Cell Biol. 1996 Mar;132(5):769-85. doi: 10.1083/jcb.132.5.769. J Cell Biol. 1996. PMID: 8603911 Free PMC article. - Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway.
Le Borgne R, Hoflack B. Le Borgne R, et al. Curr Opin Cell Biol. 1998 Aug;10(4):499-503. doi: 10.1016/s0955-0674(98)80065-3. Curr Opin Cell Biol. 1998. PMID: 9719871 Review. - Protein transport from the secretory to the endocytic pathway in mammalian cells.
Le Borgne R, Hoflack B. Le Borgne R, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):195-209. doi: 10.1016/s0167-4889(98)00057-3. Biochim Biophys Acta. 1998. PMID: 9714803 Review.
Cited by
- The AP2 clathrin adaptor protein complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of Caenorhabditis elegans.
Garafalo SD, Luth ES, Moss BJ, Monteiro MI, Malkin E, Juo P. Garafalo SD, et al. Mol Biol Cell. 2015 May 15;26(10):1887-900. doi: 10.1091/mbc.E14-06-1048. Epub 2015 Mar 18. Mol Biol Cell. 2015. PMID: 25788288 Free PMC article. - Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake.
Kasprowicz J, Kuenen S, Miskiewicz K, Habets RL, Smitz L, Verstreken P. Kasprowicz J, et al. J Cell Biol. 2008 Sep 8;182(5):1007-16. doi: 10.1083/jcb.200804162. Epub 2008 Sep 1. J Cell Biol. 2008. PMID: 18762582 Free PMC article. - Clathrin is not required for SNX-BAR-retromer-mediated carrier formation.
McGough IJ, Cullen PJ. McGough IJ, et al. J Cell Sci. 2013 Jan 1;126(Pt 1):45-52. doi: 10.1242/jcs.112904. Epub 2012 Sep 26. J Cell Sci. 2013. PMID: 23015596 Free PMC article. - The role of clathrin in post-Golgi trafficking in Toxoplasma gondii.
Pieperhoff MS, Schmitt M, Ferguson DJ, Meissner M. Pieperhoff MS, et al. PLoS One. 2013 Oct 11;8(10):e77620. doi: 10.1371/journal.pone.0077620. eCollection 2013. PLoS One. 2013. PMID: 24147036 Free PMC article. - Early endosomes are required for major histocompatiblity complex class II transport to peptide-loading compartments.
Brachet V, Péhau-Arnaudet G, Desaymard C, Raposo G, Amigorena S. Brachet V, et al. Mol Biol Cell. 1999 Sep;10(9):2891-904. doi: 10.1091/mbc.10.9.2891. Mol Biol Cell. 1999. PMID: 10473634 Free PMC article.
References
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. - PubMed
- Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM57657/GM/NIGMS NIH HHS/United States
- TPP 97-003/HX/HSRD VA/United States
- R01 GM038093/GM/NIGMS NIH HHS/United States
- GM38093/GM/NIGMS NIH HHS/United States
- AI39152/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous