Massive expansion of antigen-specific CD8+ T cells during an acute virus infection - PubMed (original) (raw)
Massive expansion of antigen-specific CD8+ T cells during an acute virus infection
E A Butz et al. Immunity. 1998 Feb.
Abstract
During LCMV infection, CD8+ T cells expand greatly. Bystander activation has been thought to play a role because few cells score as LCMV specific in limiting dilution analysis. In contrast, we find that at least a quarter of the CD8+ cells secrete IFNgamma specifically in response to LCMV peptides at the peak of the response. Moreover, by analyzing the expansion of adoptively transferred LCMV-specific, TCR-transgenic CD8+ T cells in congenic hosts, we have determined that most of the CD8+ cell expansion is virus specific. Analysis of the effect of the monospecific TCR-transgenic T cells on the host response to three LCMV epitopes suggests that CTL precursors compete for sites on the APC in an epitope-specific fashion and that this competition determines the specificity of the response.
Figures
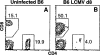
Figure 1. LCMV Infection of C57Bl/6 Mice Induces Massive Expansion of CD8+ T Cells
Representative CD4 versus CD8 flow cytometry profiles of splenocytes from uninfected (A) or day 8 LCMV-infected B6 mice (B). The numbers beside the gating boxes indicates the percentage of live-gated cells. d8, day 8.
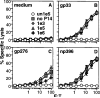
Figure 2. Antigen-Specific Cytolytic Activity Is Proportional to the Frequency of IFNγ-Secreting Cells
(A) Erythrocyte-depleted spleen cells were prepared from a pair of day 8 LCMV-infected B6 mice and were used to determine primary ex vivo CTL activity in an 8 hr 51Cr-release assay against EL4 target cells uncoated or coated with the indicated LCMV peptides. (B) The same pool of effector cells was used in an ELISPOT assay to determine the frequency of cells secreting IFNγ in response to LCMV epitope peptides. The number of CD8+ cells was determined by FACS analysis. Error bars indicate the standard deviation of triplicate samples. Three additional experiments yielded similar results. d8, day 8. (C) The titration of spots per well versus number of LCMV day 8 splenocytes per well is shown for ELISPOT data from a separate experiment. Splenocytes were stimulated in triplicate samples with either control EL4 cells (open circles) or with gp33-coated EL4 cells (filled squares). Stimulation with gp276- or np396-coated EL4 cells resulted in similarly linear responses (data not shown).
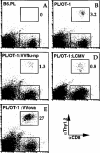
Figure 3. Adoptively Transferred TCR-Transgenic CD8+ T Cells Do Not Expand Nonspecifically during VV or LCMV Infection
Flow cytometry profiles of pooled spleen cells from pairs of B6.PL control mice (A) and B6.PL host mice that received 107 purified OT-1 CD8+ T cells and were injected the next day with PBS (B), VVflu-np (C), LCMV (D), or VVova (E). Splenocytes were analyzed on day 6 (A–C and E) or day 8 (D) of infection. The numbers beside the gating boxes indicate the number of donor CD8+ cells as a percentage of total CD8+ cells. A repetition of the experiment yielded similar results.
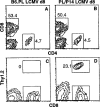
Figure 4. GP33-Specific TCR-Transgenic CD8+ T Cells Expand Dramatically during LCMV Infection
Control B6.PL mice (A and C) or B6.PL mice that had received 105 P14 spleen cells (~3 × 104 transgenic CD8+ cells) 1 day earlier (B and D) were infected with LCMV. After 8 days spleens were removed and assayed for CD4 versus CD8 staining (A and B) and donor (CD8+Thy1.2+) CTL content (C and D). The numbers beside the gating boxes in (A) and (B) indicate the percentage of live gated cells; in (C) and (D) the numbers indicate the number of Thy1.2+ donor cells as a percentage of CD8+ cells. Results are from representative individual mice from groups of three. In uninfected chime-ric mice the donor cells were less than 0.5% of CD8+ cells (data not shown). d8, day 8.
Figure 5. Adoptive Transfer of a Limited Number of gp33-Specific CD8+ T Cells Does Not Significantly Alter the Overall Response to gp33, gp276, or np396 during the Immune Response to LCMV
Primary CTL activity of splenocytes from uninfected B6.PL mice that received 105 P14 cells (open circles) and day 8 LCMV-infected mice (filled symbols) that received no P14 cells (squares), 103 P14 cells (diamonds), 105 P14 cells (triangles), or 106 P14 cells (crosses) 1 day prior to infection. Lysis was determined on day 8 of infection by 51Cr-release assay using EL4 targets without additional peptide (A) and EL4 pulsed with gp33 (B), gp276 (C), or np396 (D) peptides. Data are presented as the average specific lysis of targets by effectors from three individual mice per group, with standard deviation indicated by error bars (some of which fall within the symbols). Two repetitions of the experiment yielded similar results.
Figure 6. Both Adoptively Transferred P14 and Host CD8+ T Cells Are Functionally Active in LCMV-Infected PL/P14 Chimeras
A group of three B6.PL mice received 104 P14 spleen cells. The next day the PL/P14 chimeras (C, F, and I) and groups of three B6 (A, D, and G) and B6.PL (B, E, and H) mice were infected with LCMV. On day 8 of infection, splenocytes pooled for each group were prepared and treated with rabbit complement alone (A–C), with anti-Thy1.1 (anti-host) plus complement (D–F), or with anti-Thy1.2 (anti-donor) plus complement (G–I), and the ability of the surviving cells to lyse uncoated EL4 targets and EL4 cells coated with the indicated LCMV peptides was determined in a 51Cr-release assay.
Figure 7. Competition for MHC Class I–Peptide Complexes during CTL Priming
(Left) A virus-infected APC displays a large number of MHC class I–peptide complexes, indicated by the different numbers on the surface of the cell. (Center) When CTL precursors specific for epitopes 1 and 2 (T1 and T2) interact with the APC, they aggregate their respective target MHC–peptide complexes at the zones of contact. (Right) When enough T1 interact with the APC, they reduce the available MHC–peptide 1 complexes to the point where other T1 cells cannot be primed, although cells of other specificities (T2) can still interact productively with the APC.
Similar articles
- Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology.
Ehl S, Hombach J, Aichele P, Rülicke T, Odermatt B, Hengartner H, Zinkernagel R, Pircher H. Ehl S, et al. J Exp Med. 1998 Mar 2;187(5):763-74. doi: 10.1084/jem.187.5.763. J Exp Med. 1998. PMID: 9480986 Free PMC article. - Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response.
Zarozinski CC, Welsh RM. Zarozinski CC, et al. J Exp Med. 1997 May 5;185(9):1629-39. doi: 10.1084/jem.185.9.1629. J Exp Med. 1997. PMID: 9151900 Free PMC article. - Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection.
Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Murali-Krishna K, et al. Immunity. 1998 Feb;8(2):177-87. doi: 10.1016/s1074-7613(00)80470-7. Immunity. 1998. PMID: 9491999 - T and B cell tolerance and responses to viral antigens in transgenic mice: implications for the pathogenesis of autoimmune versus immunopathological disease.
Zinkernagel RM, Pircher HP, Ohashi P, Oehen S, Odermatt B, Mak T, Arnheiter H, Bürki K, Hengartner H. Zinkernagel RM, et al. Immunol Rev. 1991 Aug;122:133-71. doi: 10.1111/j.1600-065x.1991.tb00601.x. Immunol Rev. 1991. PMID: 1937540 Review. - The great balancing act: regulation and fate of antiviral T-cell interactions.
Moseman EA, McGavern DB. Moseman EA, et al. Immunol Rev. 2013 Sep;255(1):110-24. doi: 10.1111/imr.12093. Immunol Rev. 2013. PMID: 23947351 Free PMC article. Review.
Cited by
- Protecting and rescuing the effectors: roles of differentiation and survival in the control of memory T cell development.
Kurtulus S, Tripathi P, Hildeman DA. Kurtulus S, et al. Front Immunol. 2013 Jan 23;3:404. doi: 10.3389/fimmu.2012.00404. eCollection 2012. Front Immunol. 2013. PMID: 23346085 Free PMC article. - miRNA profiling of naïve, effector and memory CD8 T cells.
Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, Manjunath N. Wu H, et al. PLoS One. 2007 Oct 10;2(10):e1020. doi: 10.1371/journal.pone.0001020. PLoS One. 2007. PMID: 17925868 Free PMC article. - Analysis of A47, an immunoprevalent protein of vaccinia virus, leads to a reevaluation of the total antiviral CD8+ T cell response.
Yuen TJ, Flesch IE, Hollett NA, Dobson BM, Russell TA, Fahrer AM, Tscharke DC. Yuen TJ, et al. J Virol. 2010 Oct;84(19):10220-9. doi: 10.1128/JVI.01281-10. Epub 2010 Jul 28. J Virol. 2010. PMID: 20668091 Free PMC article. - Sepsis-induced changes in differentiation, maintenance, and function of memory CD8 T cell subsets.
Heidarian M, Griffith TS, Badovinac VP. Heidarian M, et al. Front Immunol. 2023 Jan 23;14:1130009. doi: 10.3389/fimmu.2023.1130009. eCollection 2023. Front Immunol. 2023. PMID: 36756117 Free PMC article. Review. - Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo.
Nishimura H, Yajima T, Naiki Y, Tsunobuchi H, Umemura M, Itano K, Matsuguchi T, Suzuki M, Ohashi PS, Yoshikai Y. Nishimura H, et al. J Exp Med. 2000 Jan 3;191(1):157-70. doi: 10.1084/jem.191.1.157. J Exp Med. 2000. PMID: 10620614 Free PMC article.
References
- Andersson EC, Christensen JP, Scheynius A, Marker O, Thomsen AR. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand J Immunol. 1995;42:110–118. - PubMed
- Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. - PubMed
- Beverley PCL. Generation of T-cell memory. Current Opinion in Immunology. 1996;8:327–330. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials