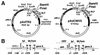Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette - PubMed (original) (raw)
Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette
B Massie et al. J Virol. 1998 Mar.
Abstract
We have constructed two new adenovirus expression cassettes that expand both the range of genes which can be expressed with adenovirus vectors (AdV) and the range of cells in which high-level expression can be attained. By inclusion of a tetracycline-regulated promoter in the transfer vector pAdTR5, it is now possible to generate recombinant adenoviruses expressing proteins that are either cytotoxic or that interfere with adenovirus replication. We have used this strategy to generate a recombinant adenovirus encoding a deletion in the R1 subunit [R1(delta2-357)] of the herpes simplex virus type 2 ribonucleotide reductase. Cell lines expressing the tetracycline-regulated transactivator (tTA) from an integrated vector or following infection with an AdV expressing tTA are able to produce deltaR1 protein at a level approaching 10% total cell protein (TCP) when infected with Ad5TR5 deltaR1 before they subsequently die. To our knowledge, this is the first report of the overexpression of a toxic gene product with AdV. We have also constructed a new constitutive adenovirus expression cassette based on an optimized cytomegalovirus immediate-early promoter-enhancer that allows the expression of recombinant proteins at a level greater than 20% TCP in nonpermissive cell lines. Together, these new expression cassettes significantly improve the utility of the adenovirus system for high-level expression of recombinant proteins in animal cells and will undoubtedly find useful applications in gene therapy.
Figures
FIG. 1
Genetic maps of pAdTR5 and pAdCMV5 transfer vectors. (A) Most of the genetic elements present in these transfer vectors have been described in detail by Massie et al. (23), and the full sequences of the plasmids are available upon request. The inner numbers on the vectors refer to map units (m.u.) on the Ad5 genome. pML2 is the E. coli replicon; the segments with dots (0 to 1 and 9.4 to 15.5 m.u.) bracketing the expression cassettes (between 1 and 9.4 m.u.) are Ad5 subgenomic portions involved in homologous recombination used to generate adenovirus recombinants. (B) Schematic representation of the expression cassettes. The expression cassettes are identical from the TATA box to the poly(A) site (pA), the main difference being upstream of the TATA box, where the tetracycline operator binding sequences (tetO) replace the CAAT box and the enhancer of the CMV major IE promoter. The diagrams are not drawn to scale. SS, splicing signal; SD, splice donor; SA, splice acceptor; tpl, tripartite leader; enh, enhancer; tet, tetracycline; Ori, origin of replication; pro, promoter. The expression cassettes contain two SDs as a result of the insertion of the MLP enhancer sequence into a small intron located downstream of the tpl in pAdBM1 (19). The first SD is the one found after the third segment of the tpl, whereas the second SD is the one found after the first segment of the tpl and was added when the MLP enhancer mapping at +30 to +130 relative to the MLP transcription start site was cloned as a fragment of 108 nucleotides (23).
FIG. 2
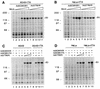
Constitutive or regulated overexpression of HSV-2 R1 in the absence of viral protein synthesis in A549 and HeLa S3 cells infected with AdV (A and B). Coomassie blue-stained gel of total proteins produced in either A549-tTA (A) or HeLa-rtTA (B) cells infected with Ad5CMV5R1 (lanes 2 to 6) or Ad5TR5R1 (lanes 7 to 11) and extracted at 48 h p.i. Both cell lines were mock infected (lane 1) or infected at MOIs of 50 (lanes 2 and 7), 100 (lanes 3 and 8), 200 (lanes 4 and 9), 400 (lanes 5 and 10), and 800 (lanes 6 and 11). Anhydrotetracycline (1 μg/ml) was added for the induction of R1 expression in HeLa-rtTA cells. (C and D) Expression of R1 compared in A549 (C) or HeLa (D) cells expressing tTA following pAdCMVtTA infection (MOI, 500) versus stable integration of the tTA or rtTA expression vectors. A549, A549-tTA, HeLa S3, or HeLa-rtTA cell lines were mock infected (lanes 1, C and D) or infected with pAdCMVtTA alone (lanes 2 and 8, C and D) or with Ad5TR5R1 at MOIs of 50 (lanes 5, C and D), 100 (lanes 6, C and D; lanes 10 in C and 9 in D), and 400 (lanes 7, C and D). Lanes 3 and 4 in C and D are control infections with Ad5TR5R1 alone at MOIs of 50 and 400, respectively, whereas lane 9 in C is an infection of A549-tTA with Ad5TR5R1 alone at an MOI of 100. For comparison, extracts of A549-tTA (lane 11 in C) or HeLa-rtTA (lane 10 in D) cells infected with Ad5CMV5R1 (MOI, 400) were loaded. Each lane was loaded with 10 μg of protein from total cell extracts. The position of R1 is indicated on the right. Molecular weight markers are shown on the left in thousands.
FIG. 3
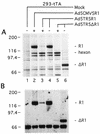
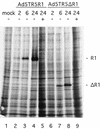
Constitutive or regulated overexpression of HSV-2 R1 and HSV-2 ΔR1 in 293-tTA cells infected with AdV. (A) Coomassie blue-stained gel of total proteins (10 μg) extracted from 293-tTA cells uninfected (lane 1) or at 48 h p.i. with Ad5CMV5R1 (lane 2), Ad5TR5R1 (lanes 3 and 4), and Ad5TR5ΔR1 (lanes 5 and 6). The positions of the R1, hexon, and ΔR1 proteins are indicated on the right. Molecular weight markers are shown on the left in thousands. + and −, with or without anhydrotetracycline (40 ng/ml), respectively. (B) Immunoblot of the same total protein extracts probed with a rabbit polyclonal antiserum raised against a 19-mer peptide corresponding to amino acids 886 to 904 of HSV-2 R1 and visualized with an ECL detection kit (Amersham). The protein band comigrating with the 66-kDa marker is bovine serum albumin, which contaminated the cell extracts. Bovine serum albumin reacted strongly with this rabbit antiserum since it (bovine serum albumin) was coupled to the R1 peptide for the immunization.
FIG. 4
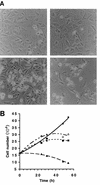
Cytoxicicity of HSV-2 ΔR1. A549-tTA cells (105 in six-well plates) were infected 24 h after seeding with 100 PFU of Ad5TR5R1 (▪), Ad5TR5ΔR1 (▴), or Ad5TR5ΔR1 plus 30 ng of anhydrotetracycline per ml (▵) per cell or were mock infected (⧫). (A) Morphological appearance of Ad5TR5R1 (top)- and Ad5TR5ΔR1 (bottom)-infected cells at 12 h (left) and 40 h (right) p.i. (B) Numbers of attached cells counted with a hemacytometer at various times p.i. (symbols are given above).
FIG. 5
Time course of HSV-2 R1 and HSV-2 ΔR1 synthesis in A549-tTA cells infected with tetracycline-regulatable AdV. A549-tTA cells were either uninfected (lane 1) or infected with Ad5TR5R1 (lanes 2 to 5) or Ad5TR5ΔR1 (lanes 6 to 9) at an MOI of 100, and total proteins were extracted following 2 h of radiolabeling with [35S]methionine at 2 h (lanes 2 and 6), 6 h (lanes 3 and 7), or 24 h (lanes 4, 5, 8, and 9) p.i. Autoradiography was performed on a gel loaded with 10 μg of protein per lane. The positions of the R1 and ΔR1 proteins are indicated on the right. + and −, with or without anhydrotetracycline (40 ng/ml).
Similar articles
- High level expression in 293 cells of the herpes simplex virus type 2 ribonucleotide reductase subunit 2 using an adenovirus vector.
Lamarche N, Massie B, Richer M, Paradis H, Langelier Y. Lamarche N, et al. J Gen Virol. 1990 Aug;71 ( Pt 8):1785-92. doi: 10.1099/0022-1317-71-8-1785. J Gen Virol. 1990. PMID: 2167932 - An improved Tet-On regulatable FasL-adenovirus vector system for lung cancer therapy.
Sipo I, Hurtado Picó A, Wang X, Eberle J, Petersen I, Weger S, Poller W, Fechner H. Sipo I, et al. J Mol Med (Berl). 2006 Mar;84(3):215-25. doi: 10.1007/s00109-005-0009-1. Epub 2005 Dec 31. J Mol Med (Berl). 2006. PMID: 16437213 - A tetracycline-regulated adenoviral expression system for in vivo delivery of transgenes to lung and liver.
Tietge UJ, Kozarsky KF, Donahee MH, Rader DJ. Tietge UJ, et al. J Gene Med. 2003 Jul;5(7):567-75. doi: 10.1002/jgm.384. J Gene Med. 2003. PMID: 12825196 - Tetracycline-regulatable adenovirus vectors: pharmacologic properties and clinical potential.
Nakagawa S, Massie B, Hawley RG. Nakagawa S, et al. Eur J Pharm Sci. 2001 Apr;13(1):53-60. doi: 10.1016/s0928-0987(00)00207-4. Eur J Pharm Sci. 2001. PMID: 11292568 Review. - Optimizing regulatable gene expression using adenoviral vectors.
Lee YB, Glover CP, Cosgrave AS, Bienemann A, Uney JB. Lee YB, et al. Exp Physiol. 2005 Jan;90(1):33-7. doi: 10.1113/expphysiol.2004.028209. Epub 2004 Nov 12. Exp Physiol. 2005. PMID: 15542617 Review.
Cited by
- The cumate gene-switch: a system for regulated expression in mammalian cells.
Mullick A, Xu Y, Warren R, Koutroumanis M, Guilbault C, Broussau S, Malenfant F, Bourget L, Lamoureux L, Lo R, Caron AW, Pilotte A, Massie B. Mullick A, et al. BMC Biotechnol. 2006 Nov 3;6:43. doi: 10.1186/1472-6750-6-43. BMC Biotechnol. 2006. PMID: 17083727 Free PMC article. - Production and Use of Gesicles for Nucleic Acid Delivery.
Mangion M, Robert MA, Slivac I, Gilbert R, Gaillet B. Mangion M, et al. Mol Biotechnol. 2022 Mar;64(3):278-292. doi: 10.1007/s12033-021-00389-6. Epub 2021 Oct 1. Mol Biotechnol. 2022. PMID: 34596870 - High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells.
Durocher Y, Perret S, Kamen A. Durocher Y, et al. Nucleic Acids Res. 2002 Jan 15;30(2):E9. doi: 10.1093/nar/30.2.e9. Nucleic Acids Res. 2002. PMID: 11788735 Free PMC article. - Nuclear translocation controlled by alternatively spliced isoforms inactivates the QUAKING apoptotic inducer.
Pilotte J, Larocque D, Richard S. Pilotte J, et al. Genes Dev. 2001 Apr 1;15(7):845-58. doi: 10.1101/gad.860301. Genes Dev. 2001. PMID: 11297509 Free PMC article. - Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis.
Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Gabai VL, et al. Mol Cell Biol. 2002 May;22(10):3415-24. doi: 10.1128/MCB.22.10.3415-3424.2002. Mol Cell Biol. 2002. PMID: 11971973 Free PMC article.
References
- Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, Karpati G. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3:579–584. - PubMed
- Acsadi G, Massie B, Jani A. Adenovirus-mediated gene transfer into striated muscles. J Mol Med. 1995;73:165–180. - PubMed
- Berkner K L. Development of adenovirus vector for the expression of heterologous genes. BioTechniques. 1988;6:616–629. - PubMed
- Berkner K L. Expression of heterologous sequences in adenoviral vectors. Curr Top Microbiol Immunol. 1992;158:39–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources