Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses - PubMed (original) (raw)
Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses
E Stein et al. Genes Dev. 1998.
Abstract
Eph family receptor tyrosine kinases (including EphA3, EphB4) direct pathfinding of neurons within migratory fields of cells expressing gradients of their membrane-bound ligands. Others (EphB1 and EphA2) direct vascular network assembly, affecting endothelial migration, capillary morphogenesis, and angiogenesis. To explore how ephrins could provide positional labels for cell targeting, we tested whether endogenous endothelial and P19 cell EphB1 (ELK) and EphB2 (Nuk) receptors discriminate between different oligomeric forms of an ephrin-B1/Fc fusion ligand. Receptor tyrosine phosphorylation was stimulated by both dimeric and clustered multimeric ephrin-B1, yet only ephrin-B1 multimers (tetramers) promoted endothelial capillary-like assembly, cell attachment, and the recruitment of low-molecular-weight phosphotyrosine phosphatase (LMW-PTP) to receptor complexes. Cell-cell contact among cells expressing both EphB1 and ephrin-B1 was required for EphB1 activation and recruitment of LMW-PTP to EphB1 complexes. The EphB1-binding site for LMW-PTP was mapped and shown to be required for tetrameric ephrin-B1 to recruit LMW-PTP and to promote attachment. Thus, distinct EphB1-signaling complexes are assembled and different cellular attachment responses are determined by a receptor switch mechanism responsive to distinct ephrin-B1 oligomers.
Figures
Figure 1
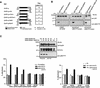
Ephrin-B1/Fc dimers and multimers elicit different responses. (A) In vitro angiogenesis assay (Martin et al. 1997) HRMEC were plated on Matrigel-coated dishes in defined medium in the absence (NA), or presence of PMA (20 ng/ml), ephrin-B1/Fc (at the indicated concentrations, ng/ml) or a control Fc fusion protein, ORF/Fc (Beckmann et al. 1994) (500 ng/ml). Fc fusion proteins were presented as either dimers, or as preclustered multimers (+ anti-Fc). Cells were photographed 8 hr after plating. (Top) Complexes recovered with EphB1 (anti-EphB1) or nonimmune (anti-NI) antibodies from HRMEC stimulated for 15 min with indicated concentrations of ephrin-B1/Fc dimers (ephrin-B1/Fc), preclustered ephrin-B1/Fc multimers (ephrin-B1/Fc plus anti-Fc) or control fusion dimers (ORF/Fc, 500 ng/ml) or multimers (ORF/Fc plus anti-Fc ng/ml), were evaluated by immunoblot for receptor recovery (anti-EphB1) and activation (anti-pTyr). (B) P19 cells were plated on fibronectin coated dishes in the presence of the indicated concentrations of dimeric or multimeric forms of ephrin-B1/Fc or ORF/Fc, as described in Materials and Methods. The percentage of total cells attached after 90 min is displayed. (Mean ±
s.e.m.
of three independent determinations). In the insert, EphB1 receptors were immunoprecipitated from P19 cells plated on fibronectin-coated dishes and stimulated with 500 ng/ml of the indicated agents for 20 min. EphB1 recovery (anti-EphB1) and activation (anti-pTyr) were evaluated by immunoblot.
Figure 1
Ephrin-B1/Fc dimers and multimers elicit different responses. (A) In vitro angiogenesis assay (Martin et al. 1997) HRMEC were plated on Matrigel-coated dishes in defined medium in the absence (NA), or presence of PMA (20 ng/ml), ephrin-B1/Fc (at the indicated concentrations, ng/ml) or a control Fc fusion protein, ORF/Fc (Beckmann et al. 1994) (500 ng/ml). Fc fusion proteins were presented as either dimers, or as preclustered multimers (+ anti-Fc). Cells were photographed 8 hr after plating. (Top) Complexes recovered with EphB1 (anti-EphB1) or nonimmune (anti-NI) antibodies from HRMEC stimulated for 15 min with indicated concentrations of ephrin-B1/Fc dimers (ephrin-B1/Fc), preclustered ephrin-B1/Fc multimers (ephrin-B1/Fc plus anti-Fc) or control fusion dimers (ORF/Fc, 500 ng/ml) or multimers (ORF/Fc plus anti-Fc ng/ml), were evaluated by immunoblot for receptor recovery (anti-EphB1) and activation (anti-pTyr). (B) P19 cells were plated on fibronectin coated dishes in the presence of the indicated concentrations of dimeric or multimeric forms of ephrin-B1/Fc or ORF/Fc, as described in Materials and Methods. The percentage of total cells attached after 90 min is displayed. (Mean ±
s.e.m.
of three independent determinations). In the insert, EphB1 receptors were immunoprecipitated from P19 cells plated on fibronectin-coated dishes and stimulated with 500 ng/ml of the indicated agents for 20 min. EphB1 recovery (anti-EphB1) and activation (anti-pTyr) were evaluated by immunoblot.
Figure 2
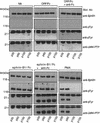
Ephrin-B1/Fc multimers recruit LMW–PTP to EphB1 and EphB2 complexes. EphB1 immunoprecipitates from HRMEC (left) or P19 cells (center) or EphB2 immunoprecipitates (from HRMEC, right) were recovered from cells exposed to no addition (NA), ORF/Fc or ephrin-B1/Fc (500 ng/ml) as either dimers (1−anti-Fc) or as preclustered multimers (+anti-Fc). Receptor complex immunoblots were analyzed to show receptor recovery (anti-EphB1), left and center, or anti-EphB2, right, receptor activation (130 kD, anti-pTyr), an 18-kD tyrosine phosphoprotein (anti-pTyr) that is recognized by antibodies to LMW–PTP (anti-LMW–PTP). The 25-kD band in all samples is immunoglobulin light chain from the immunoprecipitation. LMW–PTP is recruited to both EphB1 and EphB2 receptor complexes only by ephrin-B1/Fc multimers (+anti-Fc).
Figure 3
Reconstitution of EphB1 binding of LMW–PTP requires Y929 and an active EphB1 tyrosine kinase. (A) A yeast two-hybrid system (Stein et al. 1996) was used to analyze interactions between LMW–PTP and the wild-type EphB1 cytoplasmic domain (EphB1cy), truncations of indicated subdomains (EphB1cy/ΔJM, EphB1cy/ΔCterm) or point mutants (EphB1cyY929F, or EphB1cyK652R). Two-hybrid dependent growth (+) on histidine deficient selection media was eliminated (−) by deletion of the carboxy-terminal domain, inactivation of tyrosine-kinase function (K652R), or point mutation of Y929 (Y929F). (B) Recombinant GST fusions comprising the wild-type EphB1 cysoplasmic domain (GST–EphB1cy), a kinase-inactive mutant (GST–EphB1cyK652R) or GST–EphB1cy–Y929F/HA, were expressed in Sf9 cells, immobilized on glutathione-sepharose, incubated in kinase buffer in the presence (+) or absence (−) of ATP (Stein et al. 1996) as described in Materials and Methods. Glutathione–Sepharose-immobilized, phoshphorylated (+ATP), or unphosphorylated (−ATP) GST fusion proteins were incubated with HRMEC extracts or recombinant LMW–PTP protein, washed extensively, then analyzed by immunoblot, by use of the indicated antibodies. (C) P19 cells were transfected with the indicated ratios of pSRα expression constructs encoding wild-type EphB1 (pSRα-hEphB1/HA) or mutant (pSRα-hEphB1–Y929F/HA). Cells were stimulated with 500 ng/ml of ephrin-B1/Fc (dimers) or precomplexed ephrin-B1/Fc (multimers, +anti-Fc), or no addition, as indicated. (Top) Cells were stimulated with ephrin-B1/Fc multimers (+anti-Fc) and EphB1 immunoprecipitates (anti-HA) were analyzed for EphB1 activation (anti-pTyr) and recruitment of LMW–PTP (anti-LMW–PTP) by immunoblot. (Left) Transfected cells were exposed to indicated agonists in solution and attachment to fibronectin coated dishes was assayed (Materials and Methods). (Right) Alternatively, nitrocellulose-coated dishes were precoated with either fibronectin alone (FN), or in combination with ephrin-B1/Fc dimers or multimers (+anti-Fc), and cell attachment was assayed (Materials and Methods).
Figure 4
Ephrin-B1/Fc tetramers recruit LMW–PTP to EphB1 and promote attachment. (A) Ephrin-B1/Fc dimers (ephrin-B1/Fc, 50 μg) or preclustered multimers (ephrin-B1/Fc plus 50 μg of anti-Fc 5 μg) were separated by exclusion chromatogrpahy in PBS on a Superose 6 column (Pharmacia) precalibrated with standards of 445 kD (ferritin), 272 kD (urease), and 150 kD (IgG) as indicated. Fractions containing 500 ng/ml of protein from the indicated _A_278 peaks (A,B,C) were analyzed for activity to promote tyrosine phosphorylation of EphB1 and recruitment of LMW–PTP to EphB1 complexes (B) and to promote attachment of HRMEC and P19 cells (C). Fractions containing complexes of size consistent with tetramers (one anti-Fc molecule complexing two ephrin-B1/Fc dimers) promoted LMW–PTP recruitment and attachment.
Figure 5
Endogenous ligand activation of EphB1 requires cell–cell contact and promotes LMW–PTP recruitment. Equal numbers of HRMEC (5 × 105 cells) were plated on Matrigel-coated 35 (p35)-, 60-, (p60)-,100-, (p100)-, or 150 (p150)-mm-diam. dishes, representing 1×, 3.1×, 8.6×, or 19.5× unit surface areas, in medium supplemented with 1% bovine albumin. Cells were incubated for 2 hr at 37°C, then stimulated for 15 min with either no addition (NA), control Fc fusion (ORF/Fc, 250 ng/ml), preclustered ORF/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), ephrin-B1/Fc (250 ng/ml), preclustered ephrin-B1/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), or phorbol myristate acetate (PMA, 2 ng/ml). Immunoprecipitated EphB1 receptor complexes were analyzed for receptor activation (anti-pTyr, 130-kD band) and recruitment of the 18-kD LMW–PTP (anti-pTyr and anti-LMW–PTP). The 25-kD band is immunoglobulin light chain from the immunoprecipitation. Notably, PMA-stimulated cell density-dependent activation of EphB1 and recruitment of LMW–PTP to receptor complexes.
Figure 6
EphB1/Fc blocks juxtacrine activation of EphB1 and EphB2 by PMA and blocks capillary-like endothelial assembly. (A) HRMEC were plated on Matrigel-coated 35-mm dishes and stimulated as above with agonists added in the absence (−) or presence (+) or a competitive EphB1 ectodomain antagonist, EphB1/Fc (500 ng/ml). As before, EphB1 immunoprecipitates were analyzed for receptor recovery (anti-EphB1), activation (anti-pTyr, 130kD), and recruitment of LMW–PTP (anti-pTyr and anti-LMW–PTP, 18 kD). EphB1/Fc blocked the PMA-stimulated EphB1 activation and LMW–PTP recruitment. (B) EphB1 (left) or EphB2 (right) immunoprecipitates were recovered from HRMEC plated on Matrigel at high density and stimulated for 15 min with no addition (NA), PMA (20 ng/ml), or preclustered ephrin-B1/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), as above, in the absence (−) or presence (+) or EphB1/Fc (500 ng/ml). (C) Cells were plated as in Fig. 1 in medium containing no addition (no addition), PMA (20 ng/ml) or preclustered ephrin-B1/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), in the absence (NA) or presence (+EphB1/Fc, 500 ng/ml) of receptor ectodomain competitor. EphB1/Fc blocked capillary-like endothelial assembly responses to PMA and clustered ephrin-B1/Fc.
Similar articles
- Nck recruitment to Eph receptor, EphB1/ELK, couples ligand activation to c-Jun kinase.
Stein E, Huynh-Do U, Lane AA, Cerretti DP, Daniel TO. Stein E, et al. J Biol Chem. 1998 Jan 16;273(3):1303-8. doi: 10.1074/jbc.273.3.1303. J Biol Chem. 1998. PMID: 9430661 - Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins.
Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO. Huynh-Do U, et al. EMBO J. 1999 Apr 15;18(8):2165-73. doi: 10.1093/emboj/18.8.2165. EMBO J. 1999. PMID: 10205170 Free PMC article. - Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis.
Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J, Daniel TO. Huynh-Do U, et al. J Cell Sci. 2002 Aug 1;115(Pt 15):3073-81. doi: 10.1242/jcs.115.15.3073. J Cell Sci. 2002. PMID: 12118063 - Eph receptors and ephrins: regulators of guidance and assembly.
Wilkinson DG. Wilkinson DG. Int Rev Cytol. 2000;196:177-244. doi: 10.1016/s0074-7696(00)96005-4. Int Rev Cytol. 2000. PMID: 10730216 Review. - Signaling by Eph receptors and their ephrin ligands.
Brückner K, Klein R. Brückner K, et al. Curr Opin Neurobiol. 1998 Jun;8(3):375-82. doi: 10.1016/s0959-4388(98)80064-0. Curr Opin Neurobiol. 1998. PMID: 9687349 Review.
Cited by
- Eph receptor signaling in C. elegans.
Miller MA, Chin-Sang ID. Miller MA, et al. WormBook. 2012 Nov 29:1-17. doi: 10.1895/wormbook.1.151.1. WormBook. 2012. PMID: 23197476 Free PMC article. Review. - Deletion of low molecular weight protein tyrosine phosphatase (Acp1) protects against stress-induced cardiomyopathy.
Wade F, Quijada P, Al-Haffar KM, Awad SM, Kunhi M, Toko H, Marashly Q, Belhaj K, Zahid I, Al-Mohanna F, Stanford SM, Alvarez R, Liu Y, Colak D, Jordan MC, Roos KP, Assiri A, Al-Habeeb W, Sussman M, Bottini N, Poizat C. Wade F, et al. J Pathol. 2015 Dec;237(4):482-94. doi: 10.1002/path.4594. Epub 2015 Sep 1. J Pathol. 2015. PMID: 26213100 Free PMC article. - Eph receptors and ephrins in the developing chick cerebellum: relationship to sagittal patterning and granule cell migration.
Karam SD, Burrows RC, Logan C, Koblar S, Pasquale EB, Bothwell M. Karam SD, et al. J Neurosci. 2000 Sep 1;20(17):6488-500. doi: 10.1523/JNEUROSCI.20-17-06488.2000. J Neurosci. 2000. PMID: 10964955 Free PMC article. - Cardiovascular genomics: a current overview of in vivo and in vitro studies.
Mariappan D, Winkler J, Hescheler J, Sachinidis A. Mariappan D, et al. Stem Cell Rev. 2006;2(1):59-66. doi: 10.1007/s12015-006-0010-2. Stem Cell Rev. 2006. PMID: 17142888 Free PMC article. Review. - Prion and doppel proteins bind to granule cells of the cerebellum.
Legname G, Nelken P, Guan Z, Kanyo ZF, DeArmond SJ, Prusiner SB. Legname G, et al. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16285-90. doi: 10.1073/pnas.242611999. Epub 2002 Nov 21. Proc Natl Acad Sci U S A. 2002. PMID: 12446843 Free PMC article.
References
- Bain G, Ray WJ, Yao M, Gottlieb DI. From embryonal carcinoma cells to neurons: The P19 pathway. BioEssays. 1994;15:343–348. - PubMed
- Bohme B, VandenBos T, Cerretti DP, Park LS, Hotrich U, Ruebsamen-Waigmann H, Strebhardt K. Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem. 1996;271:24747–24752. - PubMed
- Brambilla R, Brueckner K, Orioli D, Bergemann AD, Flanagan JG, Klein R. Similarities and differences in the way transmembrane-type ligands interact with the ELK subclass of Eph receptors. Mol Cell Neurosci. 1996;8:199–209. - PubMed
- Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer F, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM27003/GM/NIGMS NIH HHS/United States
- DK47078/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- DK38517/DK/NIDDK NIH HHS/United States
- R01 DK038517/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous