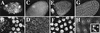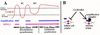Cell cycle control of chorion gene amplification - PubMed (original) (raw)
Cell cycle control of chorion gene amplification
B R Calvi et al. Genes Dev. 1998.
Abstract
Over-replication of two clusters of chorion genes in Drosophila ovarian follicle cells is essential for rapid eggshell biosynthesis. The relationship of this amplification to the follicle cell cycles has remained unclear. To investigate the regulation of amplification, we developed a technique to detect amplifying chorion genes in individual follicle cells using BrdU incorporation and FISH. Amplification occurs in two developmental phases. One of the gene clusters begins to amplify periodically during S phases of follicle cell endocycles. Subsequently, after endocycles have ceased, both clusters amplify continuously during the remainder of oogenesis. In contrast to the early phase, late amplification commences synchronously among follicle cells. The pattern of Cyclin E expression mirrors these two phases. We present evidence that Cyclin E is required positively for amplification. We suggest that Cyclin E also acts negatively to inhibit refiring of most origins within a cycle, and that specific factors at chorion origins allow them to escape this negative rereplication control. Our findings suggest that chorion amplification is a model for understanding metazoan replicons and the controls that restrict replication to once per cell cycle.
Figures
Figure 1
Three phases of follicle cell development during oogenesis. (A) ∼650 follicle cells form an epithelial layer that surrounds each maturing Drosophila egg chamber; in late stages most surround the oocyte, although a few follicle cells sheath the 15 nurse cells. Within follicle cells over the oocyte, two clusters of chorion genes on the X and third chromosomes amplify 15- and 60-fold, respectively. (B) Schematic representation of an ovariole is shown. Egg chambers originate in the germarium and migrate posteriorly down the ovariole as they develop. Follicle cells originate from two somatic stem cells in the germarium and complete approximately eight mitotic divisions over a 6-day period to achieve their final number during stage 6 (S6). Thereafter, follicle cells undergo three endocycles (see C); the exact stage at which endocycles are complete is uncertain (broken vertical line). Follicle cells then enter a postendocycle stage for the remainder of oogenesis. Amplification is known to occur during this phase (bottom solid line), but whether it initiates during the endocycles is not known (broken line). (C) FACS profile of follicle cell nuclear DNA content, showing 4C, 8C, and 16C classes produced by three endocycles. Egg chamber drawings modified from King (1970).
Figure 2
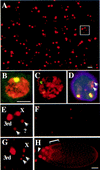
BrdU incorporation associated with endocycles and amplification. (A) In this stage 9 egg chamber, BrdU incorporation reveals that follicle cell endocycles are not synchronized. Some follicle cell nuclei are positive for BrdU incorporation and are in S, whereas others are blank for BrdU incorporation and, therefore, in G phase (see below). (B) Higher magnification to show the difference between S- and G-phase labeling. (C) DAPI of B. The DAPI bright dots correspond to follicle cell chromocenters that contain peri-centric heterochromatin. (D,E) Stage 10A chamber with the last few cells completing endocycles incorporating BrdU. The number of BrdU-positive cells decreases during mid-stage 9–10A as cells complete endocycles at different times. By late stage 10A, some chambers are entirely blank for BrdU incorporation. (F) DAPI of panel E. (G,H) In stage 10B, within hours after asynchronous completion of endocycles, punctate BrdU incorporation appears synchronously in every follicle cell over the oocyte but not those over the nurse cells (arrowhead). Notice the extreme difference between the BrdU incorporation in H and the absence of labeling in nuclei only a few hours earlier in E. (I) DAPI of H.
Figure 3
Amplification detected by BrdU and FISH. (A) Whole mount BrdU labeling in stage 10B follicle cells. Most nuclei have four spots of different intensities: one bright, one intermediate, and two faint. (B) FISH labeling. Hybridization of a chorion probe from the third chromosome to a squashed amplifying follicle cell nucleus. Total DNA is in red and hybridization signal is in yellow. (C) FISH with a chorion probe from the X chromosome to a squashed follicle cell that gives a less intense signal than the third locus commensurate with lower levels of amplification. (D) FISH/BrdU double labeling. Detection of BrdU incorporation (red) and FISH with both X and third chorion probes (green, overlap, yellow) indicating the bright BrdU focus corresponds to the highly amplified third chromosome chorion locus, the intermediate BrdU labeling to the less amplified X locus, and the two fainter spots to neither locus (arrows). The very faint extra BrdU labeling present in the center of this nucleus was not reproducibly observed. (E) Enlarged nucleus from whole mount BrdU labeling in A (white box), showing the four spot patterns and their corresponding identities. (F) Whole mount of BrdU incorporation in ovaries mutant for the Drosophila homolog of ORC2 [_fs(3)293_]. Compare with same magnification of wild type in A. Here, BrdU incorporation is mosaic in intensity among cells and is most easily seen at the third chorion locus. (G) BrdU incorporation in a stage 12 follicle cell. Notice that incorporation at the third locus is greater than earlier stages, X locus incorporation is reduced. (H) A dorsolateral aspect of a stage 11 egg chamber demonstrating brighter incorporation in the dorsal–anterior cells (bracket). BrdU foci are absent from follicle cells that remain over the nurse cells (for example, see arrowhead). The larger nuclei in the anterior of the chamber are those of the nurse cells. Scale bar in A for A and F, 5 μm; and in B for B_–_E and G, 5 μm; in H, 50 μm.
Figure 4
Amplification begins during endocycles. (A) Representative Southern blot of _Sal_I digested genomic DNA comparing the relative copy numbers of a 3.8-kb _Sal_I fragment from the third chromosome chorion gene locus (ch) to a 7.3-kb _Sal_I fragment from the rosy locus as a control (c). Chorion DNA is amplified two-fold in 8C endocycling follicle cells (Table 1). (Lane 1–3) Decreasing loadings of male genomic DNA that served as a nonamplifying diploid control; (lane 4) DNA from 8C flow-sorted ovarian nuclei (see Fig. 1C); (lane 5) DNA from 16C flow sorted ovarian nuclei. (B) Similar analysis of copy number in stage 10A egg chambers indicates third chromosome chorion genes are amplified fourfold by this stage (Table 1). Notice that the measured level in B needs to be corrected for the fraction of DNA derived from follicle cells whereas that in A does not. (Lanes 1–3) Decreasing loadings male diploid control; (lane 4) stage 10A DNA. Molecular weights of marker DNAs are indicated to left in A and B (in kb).
Figure 5
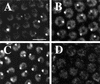
Changes in CycE behavior during the two phases of amplification. CycE protein levels within follicle cells were determined by confocal immunofluorescence. (A,B) Nuclear CycE levels oscillate during asynchronous follicle cell endocycles. (C,D) Stage 10A chamber showing that after completion of the final endocycle CycE levels drop, but a low level of staining remains. The area of less intense staining in the nuclei of this and later stages corresponds to the large nucleolus in these cells. (E,F) Shortly thereafter in stage 10, CycE rises to high levels in every follicle cell over the oocyte. The large patches of staining in A, C, and E correspond to nurse cell and oocyte nuclei. (G,H) Throughout later amplification, CycE levels slowly diminish but fail to cycle. Shown here is a stage 12 chamber demonstrating the 4 spot punctate labeling reminiscent of BrdU incorporation (inset in H, single nucleus).
Figure 6
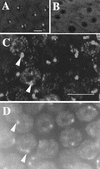
CycE is necessary, but not sufficient, for amplification. (A) BrdU foci in wild-type stage 12 nuclei. (B) Stage 12 chamber from a c323GAL4; UAS:Dap female showing that misexpression of the CycE inhibitor, Dacapo, drastically reduces BrdU labeling. (C) Overexpression of CycE in stage 10A. High magnification shows robust late replication corresponding to heterochromatic chromocenter but no BrdU incorporation characteristic of amplification. (D) DAPI image of C to show correspondence of labeling with DAPI bright chromocenter (cf. arrowheads in C and D). Scale bar, 10 μm.
Figure 7
MPM-2 reveals synchronization of the cell cycle and responds to CycE. (A) Endocycling follicle cells have cell cycle-dependent staining of an MPM-2 reactive nuclear sphere. (B) A field of cells from stage 10A. The number of MPM-2 sphere containing cells increases throughout stage 9 so that by stage 10 every follicle cell is positive. (C) Overexpression of CycE with a hsp70:cycE transgene results in an increase in the number of stage 8 endocycling follicle cells that are MPM-2 positive. (D) Stage 10 follicle cells showing that inhibition of CycE activity in hsp70:GAL4; UAS:Dap flies results in dramatic reductions in MPM-2 sphere staining. Scale bar, 10 μm.
Figure 8
A model for the two phases of amplification and escape from rereplication control. (A) In the early phase, third chorion genes amplify periodically during follicle cell endocycle S phases occurring asynchronously among cells within an egg chamber. In the late phase, chorion amplification commences synchronously among cells and replicates continuously (blue line). During the end of the early phase, follicle cells acquire synchrony as they complete the final endoreplication, but do not enter a subsequent normal endocycle. These changes in periodicity are associated with alterations in behavior of CycE oscillations (red waves above) and MPM-2 reactivity (red lines below). CycE levels must be greater than the S-phase threshold for replication (top broken line). CycE levels also must decline below the reset threshold for origins to reset (bottom broken line). If CycE levels remain above this threshold after the final endoreplication, it would explain the absence of widespread origin firing when late phase amplification begins. (B) For replication from most follicle cell origins CycE likely plays dual roles—S-phase promotion and rereplication inhibition. Here, after initiation of replication, some proteins (pink ovals) remain associated with follicle cell origins, whereas association of others (blue ovals) into prereplication complexes is inhibited by CycE. Amplification complexes resident at chorion origins escape inhibition by CycE but require its positive activity to initiate replication. It may be that in addition to proteins common to other origins, proteins specific to amplification complexes (black polygon) impart resistance to CycE inhibition.
Similar articles
- Drosophila E2f2 promotes the conversion from genomic DNA replication to gene amplification in ovarian follicle cells.
Cayirlioglu P, Bonnette PC, Dickson MR, Duronio RJ. Cayirlioglu P, et al. Development. 2001 Dec;128(24):5085-98. doi: 10.1242/dev.128.24.5085. Development. 2001. PMID: 11748144 - Chorion gene amplification in Drosophila: A model for metazoan origins of DNA replication and S-phase control.
Calvi BR, Spradling AC. Calvi BR, et al. Methods. 1999 Jul;18(3):407-17. doi: 10.1006/meth.1999.0799. Methods. 1999. PMID: 10455001 - The ecdysone regulatory cascade and ovarian development in lepidopteran insects: insights from the silkmoth paradigm.
Swevers L, Iatrou K. Swevers L, et al. Insect Biochem Mol Biol. 2003 Dec;33(12):1285-97. doi: 10.1016/j.ibmb.2003.06.012. Insect Biochem Mol Biol. 2003. PMID: 14599500 Review. - Drosophila chorion genes: cracking the eggshell's secrets.
Orr-Weaver TL. Orr-Weaver TL. Bioessays. 1991 Mar;13(3):97-105. doi: 10.1002/bies.950130302. Bioessays. 1991. PMID: 1908228 Review.
Cited by
- Localisation of the DmCdc45 DNA replication factor in the mitotic cycle and during chorion gene amplification.
Loebel D, Huikeshoven H, Cotterill S. Loebel D, et al. Nucleic Acids Res. 2000 Oct 15;28(20):3897-903. doi: 10.1093/nar/28.20.3897. Nucleic Acids Res. 2000. PMID: 11024168 Free PMC article. - Drosophila ORC specifically binds to ACE3, an origin of DNA replication control element.
Austin RJ, Orr-Weaver TL, Bell SP. Austin RJ, et al. Genes Dev. 1999 Oct 15;13(20):2639-49. doi: 10.1101/gad.13.20.2639. Genes Dev. 1999. PMID: 10541550 Free PMC article. - The Drosophila Geminin homolog: roles for Geminin in limiting DNA replication, in anaphase and in neurogenesis.
Quinn LM, Herr A, McGarry TJ, Richardson H. Quinn LM, et al. Genes Dev. 2001 Oct 15;15(20):2741-54. doi: 10.1101/gad.916201. Genes Dev. 2001. PMID: 11641279 Free PMC article. - Dm-myb mutant lethality in Drosophila is dependent upon mip130: positive and negative regulation of DNA replication.
Beall EL, Bell M, Georlette D, Botchan MR. Beall EL, et al. Genes Dev. 2004 Jul 15;18(14):1667-80. doi: 10.1101/gad.1206604. Genes Dev. 2004. PMID: 15256498 Free PMC article. - The molecular chaperone Hsp90 is required for cell cycle exit in Drosophila melanogaster.
Bandura JL, Jiang H, Nickerson DW, Edgar BA. Bandura JL, et al. PLoS Genet. 2013;9(9):e1003835. doi: 10.1371/journal.pgen.1003835. Epub 2013 Sep 26. PLoS Genet. 2013. PMID: 24086162 Free PMC article.
References
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
- Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. - PubMed
- Carminati JL, Orr-Weaver TL. Changes in DNA replication during animal development. In: DePamphilis ML, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 409–434.
- Dahmann C, Diffley JFX, Nasmyth KA. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transistion of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases