Identification of an activation region in the proteasome activator REGalpha - PubMed (original) (raw)
Comparative Study
Identification of an activation region in the proteasome activator REGalpha
Z Zhang et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Proteasomes can be markedly activated by associating with 19S regulatory complexes to form the 26S protease or by binding 11S protein complexes known as REG or PA28. Three REG subunits, alpha, beta, and gamma, have been expressed in Escherichia coli, and each recombinant protein can activate human proteasomes. Combining PCR mutagenesis with an in vitro activity assay, we have isolated and characterized 36 inactive, single-site mutants of recombinant REGalpha. Most are monomers that produce functional proteasome activators when mixed with REGbeta subunits. Five REGalpha mutants that remain inactive in the mixing assay contain amino acid substitutions clustered between Arg-141 and Gly-149. The crystal structure of the REGalpha heptamer shows that this region forms a loop at the base of each REGalpha subunit. One mutation in this loop (N146Y) yields a REGalpha heptamer that binds the proteasome as tightly as wild-type REGalpha but does not activate peptide hydrolysis. Corresponding amino acid substitutions in REGbeta (N135Y) and REGgamma (N151Y) produce inactive proteins that also bind the proteasome and inhibit proteasome activation by their normal counterparts. Our studies clearly demonstrate that REG binding to the proteasome can be separated from activation of the enzyme. Moreover, the dominant negative REGs identified here should prove valuable for elucidating the role(s) of these proteins in antigen presentation.
Figures
Figure 1
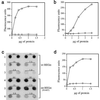
Activity screen for REGα mutants. The two panels show a small section of a 96-well ELISA plate that illustrates the enzyme assay (Left) and the antibody screen for REGα expression (Right). Wells marked with white asterisks identify inactive REGα variants. The fluorescence shown in the positive wells is similar to that in wells containing bacteria that express wild-type REGα as control. In addition, the use of antibodies to detect REGα variants may account for the paucity of folding mutants in our collection.
Figure 2
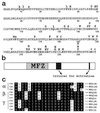
Inactivating single-site mutations in REGα. (a) Single-site changes that produce completely inactive variants are indicated by bold capital letters placed above the wild-type sequence, which is presented in the one-letter code (50 residues per line). Nine amino acid substitutions that produce partially active variants are denoted by lowercase letters. (b) The sequence of REGα is depicted as an elongated rectangle in which the mutation free zone (MFZ) is identified by stippling, and the positions of residues important for proteasome activation are shown in black. (c) Sequence alignments of the region most highly conserved among REG homologs. These sequences contain the blackened activation region, Arg-141 to Gly-149, shown near the center of b and which appears to possess a suitable geometry for interaction with the proteasome (24). The letters at the far right identify the species: h, human; m, mouse; r, rat; t, tick; b, bovine.
Figure 3
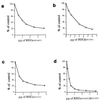
Importance of a conserved asparagine for proteasome activation by REG homologs. (a) Proteasome stimulation by recombinant REGα (squares) and rREGαN146Y (diamonds). Human red cell proteasome (170 ng) was mixed with increasing amounts of REGα or rREGαN146Y to a final volume of 50 μl, incubated at 37°C for 10 min, and then 50 μl of 200 μM Suc-LLVY-MCA was added. After 10 min the reaction was quenched by using 200 μl of 100% ethanol, and enzyme activity is reported as fluorescence units. (b) Proteasome stimulation by REGβ (squares) and REGβN135Y (diamonds). This experiment was performed as described in a. (c) Proteasome binding properties of rREGα and rREGαN146Y. After tethering proteasomes to ELISA plates with anti-proteasome antibody, different concentrations of rREGα or rREGαN146Y were added to each well and incubated. Bound REG was eluted with 0.5 M KCl, blotted onto nitrocellulose, and detected with rabbit anti-REGα (see Methods). The number above each pair of dot blots indicates the incubation concentration of REGs in μg/ml. The apparent tighter binding exhibited by REGα(N146Y) is due to the fact that anti REGα serum detects the mutant protein better than it detects wild-type REGα (data not shown). (d) Proteasome stimulation by REGγ (squares) and REGγN151Y (diamonds). The assays were done in a except that Suc-LRR-MCA was used as substrate. Each data point in a, b, and d represents the mean of three measurements from a single experiment. But equivalent results were observed in at least two experiments by using different preparations of the various REG proteins.
Figure 4
Dominant negative properties of REG homologs with Asn-to-Tyr substitutions. (a) Inhibition of REGα-activated peptide hydrolysis by REGαN146Y. Proteasomes (170 ng) and 1.5 μg of REGα were mixed with increasing amounts of REGαN146Y, incubated at 37°C for 10 min, and then 50 μl of 200 μM Suc-LLVY-MCA was added to start the reactions. Product formation was measured as fluorescence units, and the results are expressed as a percentage of the activity in the absence of REGαN146Y. (b) Inhibition of REGβ’s activity by REGβN135Y. The assay was performed as described in a except that 3 μg of REGβ was added to each reaction. (c) Inhibition of REGγ’s activity by REGγN151Y. The assay was performed as described in a except that Suc-LRR-MCA was the substrate. (d) Inhibition of the activity of REG from human red blood cells by REGαN146Y/REGβN135Y. REGαN146Y/REGβN135Y heterooligomers were preformed by overnight incubation at 4°C and purified by gel filtration chromatography. Hrbc REG (1.5 μg) was added to each reaction, and increasing amounts of REGαN146Y/REGβN135Y were added prior to substrate. The enzymatic reaction was then performed as in a. Less inhibition was observed when recombinant wild-type REGα/β oligomers were challenged with REGαN146Y/REGβN135Y. Each point in all the figures represents the mean of three measurements. All inhibition experiments were performed independently at least twice by using REG proteins from different purifications.
Figure 5
Location of inactive mutations in the rREGα crystal structure. (a) One rREGα monomer is shown in yellow within the assembled heptamer. (b) The monomer backbone is shown in yellow with the side chains of mutated residues. This model stops at residue 241 because the last 8 residues are disordered in the crystal structure. Residues that block proteasome binding and/or activation are shown in red and labeled explicitly. Mutants that block heptamer formation are in blue. Also in blue are residues Ser-175 and Ala-224, which, when mutated, produce heptamers that fail to bind the proteasome; we speculate that these mutations result in a distorted heptameric conformation. The more severe “folding” mutant residues, Arg-181 and Asp-205, which form a buried salt bridge, are shown in orange. The ends of the disordered 39-residue loop are indicated with asterisks.
Comment in
- The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death.
Ciechanover A, Schwartz AL. Ciechanover A, et al. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):2727-30. doi: 10.1073/pnas.95.6.2727. Proc Natl Acad Sci U S A. 1998. PMID: 9501156 Free PMC article. No abstract available.
Similar articles
- Characterization of recombinant REGalpha, REGbeta, and REGgamma proteasome activators.
Realini C, Jensen CC, Zhang Z, Johnston SC, Knowlton JR, Hill CP, Rechsteiner M. Realini C, et al. J Biol Chem. 1997 Oct 10;272(41):25483-92. doi: 10.1074/jbc.272.41.25483. J Biol Chem. 1997. PMID: 9325261 - The proteasome activator 11 S regulator or PA28. Contribution By both alpha and beta subunits to proteasome activation.
Zhang Z, Clawson A, Rechsteiner M. Zhang Z, et al. J Biol Chem. 1998 Nov 13;273(46):30660-8. doi: 10.1074/jbc.273.46.30660. J Biol Chem. 1998. PMID: 9804839 - The proteasome activator 11 S REG (PA28) and class I antigen presentation.
Rechsteiner M, Realini C, Ustrell V. Rechsteiner M, et al. Biochem J. 2000 Jan 1;345 Pt 1(Pt 1):1-15. Biochem J. 2000. PMID: 10600633 Free PMC article. Review. - Molecular dissection of the 11S REG (PA28) proteasome activators.
Li J, Rechsteiner M. Li J, et al. Biochimie. 2001 Mar-Apr;83(3-4):373-83. doi: 10.1016/s0300-9084(01)01236-6. Biochimie. 2001. PMID: 11295500 Review.
Cited by
- The molecular basis of wound healing processes induced by lithospermi radix: a proteomics and biochemical analysis.
Hsiao CY, Tsai TH, Chak KF. Hsiao CY, et al. Evid Based Complement Alternat Med. 2012;2012:508972. doi: 10.1155/2012/508972. Epub 2012 Sep 17. Evid Based Complement Alternat Med. 2012. PMID: 23024692 Free PMC article. - The ubiquitin-proteasome system.
Nandi D, Tahiliani P, Kumar A, Chandu D. Nandi D, et al. J Biosci. 2006 Mar;31(1):137-55. doi: 10.1007/BF02705243. J Biosci. 2006. PMID: 16595883 Review. - Regulation of c-Myc protein stability by proteasome activator REGγ.
Li S, Jiang C, Pan J, Wang X, Jin J, Zhao L, Pan W, Liao G, Cai X, Li X, Xiao J, Jiang J, Wang P. Li S, et al. Cell Death Differ. 2015 Jun;22(6):1000-11. doi: 10.1038/cdd.2014.188. Epub 2014 Nov 21. Cell Death Differ. 2015. PMID: 25412630 Free PMC article. - Tetra-anionic porphyrin mimics protein-protein interactions between regulatory particles and the catalytic core, allosterically activating human 20S proteasome.
Santoro AM, Persico M, D'Urso A, Cunsolo A, Tkachuk O, Milardi D, Purrello R, Tundo GR, Sbardella D, Osmulski PA, Gaczynska M, Coletta M, Fattorusso C. Santoro AM, et al. J Enzyme Inhib Med Chem. 2025 Dec;40(1):2482892. doi: 10.1080/14756366.2025.2482892. Epub 2025 Apr 7. J Enzyme Inhib Med Chem. 2025. PMID: 40192126 Free PMC article. - Interactions of PAN's C-termini with archaeal 20S proteasome and implications for the eukaryotic proteasome-ATPase interactions.
Yu Y, Smith DM, Kim HM, Rodriguez V, Goldberg AL, Cheng Y. Yu Y, et al. EMBO J. 2010 Feb 3;29(3):692-702. doi: 10.1038/emboj.2009.382. Epub 2009 Dec 17. EMBO J. 2010. PMID: 20019667 Free PMC article.
References
- Yewdell J W, Bennink J R. Adv Immunol. 1992;52:1–123. - PubMed
- Heemels M-H, Ploegh H. Annu Rev Biochem. 1995;64:463–491. - PubMed
- Groettrup M, Soza A, Kuckelhorn U, Kloetzel P-M. Immunol Today. 1996;17:429–435. - PubMed
- Tanaka K, Tanahashi N, Tsurumi C, Yokota K Y, Shimbara N. Adv Immunol. 1997;64:1–38. - PubMed
- Gross M, Ditzel L, Löwe J, Stock D, Bochtler M, Bartunik H D, Huber R. Nature (London) 1997;386:463–471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources