Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats - PubMed (original) (raw)
Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats
C Pesold et al. Proc Natl Acad Sci U S A. 1998.
Abstract
During embryonic development of brain laminated structures, the protein Reelin, secreted into the extracellular matrix of the cortex and hippocampus by Cajal-Retzius (CR) cells located in the marginal zone, contributes to the regulation of migration and positioning of cortical and hippocampal neurons that do not synthesize Reelin. Soon after birth, the CR cells decrease, and they virtually disappear during the following 3 weeks. Despite their disappearance, we can quantify Reelin mRNA (approximately 200 amol/ g of total RNA) and visualize it by in situ hybridization, and we detect the translated product of this mRNA by immunocytochemistry preferentially in gamma-aminobutyric acid (GABA)ergic neurons of adult rat cortex and hippocampus. In adult rat cerebellum, Reelin is expressed in glutamatergic neurons (granule cells). The translated product of this mRNA is readily exported from the granule cell somata to the parallel fibers, where it has been detected by electron microscopy in axon terminals located presynaptically to Purkinje cell dendrites.
Figures
Figure 1
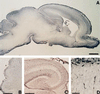
Reelin (G10)-immunostaining in the neonatal [postnatal day 0 (P0)] rat. Low magnification of a brain sagittal section (A) shows that Reelin is most abundant in the marginal zone of the cerebral cortex, hippocampus, cerebellum, and olfactory bulb. Higher magnification of the cerebellum (B) shows that Reelin is most abundant in the EGL. C shows that the highest Reelin expression is around the hippocampal fissure. D, which is a high magnification of the cortical plate boxed in A, shows that Reelin is highly expressed in large horizontally oriented fusiform neurons, presumably CR cells. [Bars = 1 mm (A), 25 μm (B and C), and 50 μm (D).]
Figure 2
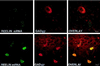
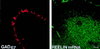
Confocal laser microscope images of Reelin mRNA detected by in situ hybridization using a biotinylated oligoprobe (base pairs 1264–1311) visualized with fluorescein (green) (Top), and GAD67 immunohistochemistry visualized with rhodamine (red) (Middle), in a hippocampal CR cell at P0. (Bottom) Overlay of Top and Middle showing that Reelin mRNA colocalizes with GAD67 (yellow). (×4,000.)
Figure 3
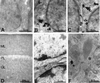
Electron micrographs of Reelin immunolabeling in rat cerebellum. (A–C) Electron micrographs of Reelin gold immunolabeling in P9 rat cerebellum. (A) Absence of gold immunolabeling in the outer part of the granule cell layer. (B) Abundance of gold immunolabeling in neuronal somata of EGL (inner part). (C) Gold immunolabeling in neuronal somata of internal granule cell layer (asterisks indicates “secreted” Reelin). (D) Light micrograph of adult rat cerebellum DAB immunostained for Reelin. Note that Reelin is abundant in the molecular (ML) and granule cell layer (GL) but absent from the Purkinje cell layer (PL). (E) Electron micrograph of the granule cell layer, showing absence of Reelin immunolabeling. (F) Electron micrograph of the molecular layer. Note that the Purkinje cell dendrite crossing the picture from bottom to top includes a large mitochondrion (asterisk). One of the neighboring parallel fibers shows Reelin gold immunolabeling (star).
Figure 4
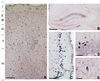
Light micrograph of adult rat brain DAB immunostained for Reelin (blue) and counterstained with neutral red for cells (red). A illustrates that Reelin-positive cells are found throughout the cortical laminae but are particularly numerous in layer V. B shows a diffuse Reelin band lining the hippocampal fissure, in addition to numerous Reelin-positive cells around this fissure, in the stratum oriens, and in the hilus of the dentate gyrus. C shows a high density of Reelin-positive cells in entorhinal cortex layer II. D is a high magnification of the boxed area of B showing Reelin-positive cells and extracellular reelin around the hippocampal fissure. E is a high magnification of the box in A illustrating Reelin expressed in somata and proximal dendrites of a cortical bitufted cell. [Bars = 150 μm (A), 500 μm (B), 125 μm (C), 50 μm (D), and 25 μm (E).]
Figure 5
Confocal microscope images of Reelin mRNA detected by in situ hybridization using a biotinylated oligoprobe (base pairs 1264–1311) visualized with fluorescein (green) and GAD67 immunolabeling visualized with rhodamine (red) in adult rat neocortex (Upper) and hippocampus (Lower). The overlay of the Reelin and GAD67 distribution shows that in the cortex mRNA for Reelin is found in both GABAergic and non-GABAergic cells, whereas in the hippocampus (CA1) all Reelin-positive cells are GABAergic. Similar results were obtained with Reelin antisense strand oligoprobe (base pairs 10225–10282).
Figure 6
Confocal microscope images of adult rat cerebellum, showing the distribution of GAD67 (immunolabeled with rhodamine: red) and Reelin mRNA (detected with biotinylated oligoprobe base pairs 1264–1311 and immunolabeled with fluorescein: green). Note that GAD67 is found predominantly in the Purkinje cell layer (Left), whereas mRNA for Reelin is found primarily in the granule cell layer (Right).
Similar articles
- Developmental distribution of a reeler gene-related antigen in the rat hippocampal formation visualized by CR-50 immunocytochemistry.
Drakew A, Frotscher M, Deller T, Ogawa M, Heimrich B. Drakew A, et al. Neuroscience. 1998 Feb;82(4):1079-86. doi: 10.1016/s0306-4522(97)00326-6. Neuroscience. 1998. PMID: 9466431 - In Patas monkey, glutamic acid decarboxylase-67 and reelin mRNA coexpression varies in a manner dependent on layers and cortical areas.
Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, Guidotti A. Rodriguez MA, et al. J Comp Neurol. 2002 Sep 23;451(3):279-88. doi: 10.1002/cne.10341. J Comp Neurol. 2002. PMID: 12210139 - Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability.
Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Costa E, et al. Neurobiol Dis. 2001 Oct;8(5):723-42. doi: 10.1006/nbdi.2001.0436. Neurobiol Dis. 2001. PMID: 11592844 Review. - Building a human cortex: the evolutionary differentiation of Cajal-Retzius cells and the cortical hem.
Meyer G. Meyer G. J Anat. 2010 Oct;217(4):334-43. doi: 10.1111/j.1469-7580.2010.01266.x. J Anat. 2010. PMID: 20626498 Free PMC article. Review.
Cited by
- Consensus paper: pathological role of the cerebellum in autism.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Fatemi SH, et al. Cerebellum. 2012 Sep;11(3):777-807. doi: 10.1007/s12311-012-0355-9. Cerebellum. 2012. PMID: 22370873 Free PMC article. Review. - Atypical mouse cerebellar development is caused by ectopic expression of the forkhead box transcription factor HNF-3beta.
Zhou H, Hughes DE, Major ML, Yoo K, Pesold C, Costa RH. Zhou H, et al. Gene Expr. 2001;9(4-5):217-36. doi: 10.3727/000000001783992597. Gene Expr. 2001. PMID: 11444531 Free PMC article. - Development of layer I neurons in the primate cerebral cortex.
Zecevic N, Rakic P. Zecevic N, et al. J Neurosci. 2001 Aug 1;21(15):5607-19. doi: 10.1523/JNEUROSCI.21-15-05607.2001. J Neurosci. 2001. PMID: 11466432 Free PMC article. - Prenatal and early life diesel exhaust exposure disrupts cortical lamina organization: Evidence for a reelin-related pathogenic pathway induced by interleukin-6.
Chang YC, Daza R, Hevner R, Costa LG, Cole TB. Chang YC, et al. Brain Behav Immun. 2019 May;78:105-115. doi: 10.1016/j.bbi.2019.01.013. Epub 2019 Jan 19. Brain Behav Immun. 2019. PMID: 30668980 Free PMC article. - Dab1 is required for synaptic plasticity and associative learning.
Trotter J, Lee GH, Kazdoba TM, Crowell B, Domogauer J, Mahoney HM, Franco SJ, Müller U, Weeber EJ, D'Arcangelo G. Trotter J, et al. J Neurosci. 2013 Sep 25;33(39):15652-68. doi: 10.1523/JNEUROSCI.2010-13.2013. J Neurosci. 2013. PMID: 24068831 Free PMC article.
References
- Rakic P, Caviness V S., Jr Neuron. 1995;14:1101–1104. - PubMed
- D’Arcangelo G, Miao G G, Chen S C, Soares H D, Morgan J L, Curran T. Nature (London) 1995;374:719–723. - PubMed
- Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, Nakao K, Katsuki M, Hayashizaki Y. Nat Genet. 1995;10:77–83. - PubMed
- Caviness V S, Rakic P. Annu Rev Neurosci. 1978;1:297–326. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources