Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function - PubMed (original) (raw)
Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function
Q Guo et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Mutations in the presenilin 1 (PS-1) gene account for many cases of early-onset autosomal dominant inherited forms of Alzheimer's disease. Recent findings suggest that PS-1 mutations may sensitize neurons to apoptosis induced by trophic factor withdrawal and exposure to amyloid beta-peptide (Abeta). We now report that overexpression of the calcium-binding protein calbindin D28k prevents apoptosis in cultured neural cells expressing mutant PS-1 (L286V and M146V missense mutations). Elevations of the intracellular Ca2+ concentration and generation of reactive oxygen species induced by Abeta, and potentiated by mutant PS-1, were suppressed in calbindin-overexpressing cells. Impairment of mitochondrial function by Abeta (which preceded apoptosis) was exacerbated by PS-1 mutations and was largely prevented by calbindin. These findings suggest that PS-1 mutations render neurons vulnerable to apoptosis by a mechanism involving destabilization of cellular calcium homeostasis, which leads to oxidative stress and mitochondrial dysfunction.
Figures
Figure 1
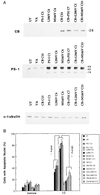
Calbindin overexpression protects PC12 cells against the proapoptotic action of mutant PS-1. (A) Representative Western blots showing levels of PS-1 and calbindin protein expression in the PC12 cell lines used. Proteins in homogenates from the indicated cell lines were separated by SDS/PAGE (50 μg of protein per lane), transferred to a nitrocellulose sheet, and immunoreacted with a polyclonal anti-calbindin antibody (Top), a polyclonal anti-PS-1 antibody (Middle), or a monoclonal anti-α-tubulin antibody (Bottom). UT, untransfected parent cell line; VA, line transfected with empty vector; PS-1C1, a line overexpressing wild-type PS-1; L286VC6, a line overexpressing the PS-1 L286V mutation; M146VC4, a line overexpressing the PS-1 M146V mutation; CB13, a line overexpressing calbindin; CB+PS1C7, a line overexpressing both wild-type PS-1 and calbindin; CB+L286VC1, a line overexpressing PS-1L286V and calbindin; and CB+M146VC12, a line overexpressing both PS-1M146V and calbindin. (B) Cultures of the indicated cell lines were exposed for 24 hr to either vehicle or 50 μM Aβ1–42. Cells were stained with Hoescht 33342, and the percentage of cells in each culture with apoptotic nuclei (condensed and fragmented DNA) was determined. Values are the mean ± SD of determinations made in four cultures (ANOVA with Scheffe’s posthoc tests).
Figure 2
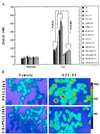
Increases of [Ca2+]i induced by Aβ are enhanced in cells expressing mutant PS-1 and attenuated by overexpression of calbindin. (A) Cells were exposed for 4 hr to 50 μM Aβ1–42 and the [Ca2+]i in individual cells was quantified by fluorescence ratio imaging of the calcium indicator dye fura-2 (see Fig. 1 for cell lines). Values are the mean ± SD of determinations made in three cultures (50–80 cells per culture; ANOVA with Scheffe’s posthoc tests). (B) Ratio images of intracellular calcium levels in PC12 cells expressing mutant PS-1 alone (PS1L286V) or in combination with calbindin (CB+PS1L286V) 4 hr after exposure to either vehicle (water) or 50 μM Aβ1–42. The [Ca2+]i is represented on a color scale shown at the right (values are nM).
Figure 3
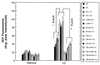
Increases of cellular reactive oxygen species induced by Aβ are enhanced in cells expressing mutant PS-1 and are prevented by overexpression of calbindin. Cells were exposed for 4 hr to 50 μM Aβ1–42 and levels of ROS in individual cells were measured by using the fluorescent probe DCF (see Fig. 1 for cell lines). Values are the mean ± SD of determinations made in three cultures (40–65 cells per culture; ANOVA with Scheffe’s posthoc tests).
Figure 4
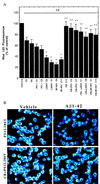
Decrease in mitochondrial transmembrane potential induced by Aβ is exacerbated in cells expressing mutant PS-1s and is prevented by overexpression of calbindin. (A) Cells were exposed for 12 hr to 50 μM Aβ1–42 and levels of rhodamine 123 fluorescence were quantified (see Fig. 1 for cell lines). Values are the mean ± SD of determinations made in three cultures (ANOVA with Scheffe’s posthoc tests). ∗, P < 0.01 compared with values for untransfected, vector-transfected, and PS-1 C1 cell lines exposed to Aβ; ∗∗, P < 0.01 and ∗∗∗, P < 0.001 compared with the value for the corresponding cell line not expressing calbindin. (B) Confocal laser scanning microscope images of rhodamine 123 fluorescence in PC12 cells expressing mutant PS-1 alone (PS1L286V) or in combination with calbindin (CB+PS1L286V) 12 hr after exposure to either vehicle (water) or 50 μM Aβ1–42. Note that Aβ1–42 caused a marked decrease in rhodamine 123 fluorescence in cells lacking calbindin but not in cells overexpressing calbindin.
Similar articles
- Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals.
Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Guo Q, et al. J Neurosci. 1997 Jun 1;17(11):4212-22. doi: 10.1523/JNEUROSCI.17-11-04212.1997. J Neurosci. 1997. PMID: 9151738 Free PMC article. - Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production.
Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Keller JN, et al. J Neurosci. 1998 Jun 15;18(12):4439-50. doi: 10.1523/JNEUROSCI.18-12-04439.1998. J Neurosci. 1998. PMID: 9614221 Free PMC article. - Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease.
Mattson MP, Guo Q, Furukawa K, Pedersen WA. Mattson MP, et al. J Neurochem. 1998 Jan;70(1):1-14. doi: 10.1046/j.1471-4159.1998.70010001.x. J Neurochem. 1998. PMID: 9422341 Review. - Cell and molecular neurobiology of presenilins: a role for the endoplasmic reticulum in the pathogenesis of Alzheimer's disease?
Mattson MP, Guo Q. Mattson MP, et al. J Neurosci Res. 1997 Nov 15;50(4):505-13. doi: 10.1002/(SICI)1097-4547(19971115)50:4<505::AID-JNR1>3.0.CO;2-I. J Neurosci Res. 1997. PMID: 9404712 Review.
Cited by
- Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice.
Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Leissring MA, et al. J Cell Biol. 2000 May 15;149(4):793-8. doi: 10.1083/jcb.149.4.793. J Cell Biol. 2000. PMID: 10811821 Free PMC article. - Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect.
Pi G, Gao D, Wu D, Wang Y, Lei H, Zeng W, Gao Y, Yu H, Xiong R, Jiang T, Li S, Wang X, Guo J, Zhang S, Yin T, He T, Ke D, Li R, Li H, Liu G, Yang X, Luo MH, Zhang X, Yang Y, Wang JZ. Pi G, et al. Nat Commun. 2020 Jan 10;11(1):183. doi: 10.1038/s41467-019-13919-3. Nat Commun. 2020. PMID: 31924799 Free PMC article. - Oxidative Stress-Mediated Brain Dehydroepiandrosterone (DHEA) Formation in Alzheimer's Disease Diagnosis.
Rammouz G, Lecanu L, Papadopoulos V. Rammouz G, et al. Front Endocrinol (Lausanne). 2011 Nov 8;2:69. doi: 10.3389/fendo.2011.00069. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654823 Free PMC article. - NF-kappaB in the survival and plasticity of neurons.
Mattson MP. Mattson MP. Neurochem Res. 2005 Jun-Jul;30(6-7):883-93. doi: 10.1007/s11064-005-6961-x. Neurochem Res. 2005. PMID: 16187223 Review.
References
- Hardy J. Trends Neurosci. 1997;20:154–159. - PubMed
- Yankner B A. Neuron. 1996;16:921–932. - PubMed
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–103. - PubMed
- Games D, Adams D, Alessandrinl R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. J Neurosci. 1992;12:376–389. - PubMed
- Mattson M P. Physiol Rev. 1997;77:1081–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010836/AG/NIA NIH HHS/United States
- AG05144/AG/NIA NIH HHS/United States
- P50 AG005144/AG/NIA NIH HHS/United States
- NS35253/NS/NINDS NIH HHS/United States
- AG14554/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous