Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion - PubMed (original) (raw)
Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion
K Honda et al. J Cell Biol. 1998.
Erratum in
- J Cell Biol 1998 Oct 5;143(1):following 276
Abstract
Regulation of the actin cytoskeleton may play a crucial role in cell motility and cancer invasion. We have produced a monoclonal antibody (NCC- Lu-632, IgM, k) reactive with an antigenic protein that is upregulated upon enhanced cell movement. The cDNA for the antigen molecule was found to encode a novel isoform of nonmuscle alpha-actinin. This isoform (designated actinin-4) was concentrated in the cytoplasm where cells were sharply extended and in cells migrating and located at the edge of cell clusters, but was absent from focal adhesion plaques or adherens junctions, where the classic isoform (actinin-1) was concentrated. Actinin-4 shifted steadily from the cytoplasm to the nucleus upon inhibition of phosphatidylinositol 3 kinase or actin depolymerization. The cytoplasmic localization of actinin-4 was closely associated with an infiltrative histological phenotype and correlated significantly with a poorer prognosis in 61 cases of breast cancer. These findings suggest that cytoplasmic actinin-4 regulates the actin cytoskeleton and increases cellular motility and that its inactivation by transfer to the nucleus abolishes the metastatic potential of human cancers.
Figures
Figure 1
Deduced amino acid sequence of actinin-4 (upper) in comparison with actinin-1 (lower). The nucleotide sequence of actinin-4 is available from GenBank/EMBL/DDBJ under accession number D89980. Asterisks represent identical amino acids and dots represent conserved amino acids. The sequences conserved among α-actinin isoforms are underlined: actin-binding domain (amino acids 111–125, single underlining), PIP2-binding domain (150–165, double underlining), and EF-hand calcium regulation domains (742–770 and 783–811, lined boxes).
Figure 2
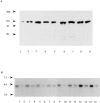
Detection of actinin-4 protein and mRNA. (A) Immunoblot analysis of actinin-4 in HFK and various human cancer cell lines with mAb NCC-Lu-632. Actinin-4 protein was detected in cell lysates from HFK (lane 1), lung cancer cell lines Lu-65 (lane 2) and PC-10 (lane 3), vulvar cancer cell line A-431 (lane 4), and esophageal cancer cell lines TE 4 (lane 5), TE 6 (lane 6), TE 7 (lane 7), TE 10 (lane 8), and TE 11 (lane 9). Molecular masses (in kD) are shown on the left. (B) Expression of actinin-4 mRNA in normal human tissues. Human multiple tissue Northern blots I and II (CLONTECH Laboratories) were hybridized with an actinin-4–specific oligonucleotide. Each lane contains 2 μg of poly(A)+ RNA of human adult tissues: heart (lane 1), brain (lane 2), placenta (lane 3), lung (lane 4), liver (lane 5), skeletal muscle (lane 6), kidney (lane 7), pancreas (lane 8), spleen (lane 9), thymus (lane 10), prostate (lane 11), testis (lane 12), ovary (lane 13), small intestine (lane 14), colon (lane 15), and peripheral blood leukocytes (lane 16). Molecular masses (in kb) are shown on the left side.
Figure 3
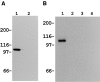
Antibody specificity to actinin-1 and -4. (A) A polypeptide of actinin-4 (amino acids 410–664) and a corresponding site of actinin-1 (amino acids 418–672) were expressed as GST fusion proteins in E. coli. Immunoblot analysis revealed that NCC- Lu-632 mAb reacts only with the actinin-4 GST fusion protein (ACTN4 GST), and not with the actinin-1 GST fusion protein (ACTN1 GST) or GST alone (GST). Molecular masses (in kD) are shown on the left. (B) Coomassie blue staining of the blot corresponding to that in A, demonstrating proper protein loading in each lane. (C) In vitro translation products of actinin-1 and -4. SDS-PAGE and autoradiography reveal 35S-labeled actinin-1 and -4 proteins. Molecular masses (in kD) are shown on the left. (D) In vitro translation products of actinin-1 (ACTN 1) and actinin-4 (ACTN 4) were immunoprecipitated by anti–chicken actinin mAb BM-75.2 or normal mouse IgM. Actinin-1 but not actinin-4 was precipitated with BM-75.2. Molecular masses (in kD) are shown on the left.
Figure 4
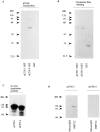
Reactivity of polyclonal antibody against actinin-4 peptide. (A) Immunoblot analysis reveals that the polyclonal antibody raised against actinin-4 peptide (lane 1) reacts with a single protein of ∼100 kD in the cell lysate of WiDr cells. A blot with normal rabbit IgG (lane 2) is included as a negative control. Molecular masses (in kD) are shown on the left. (B) In vitro translation products of actinin-4 (lanes 1 and 3) and actinin-1 (lanes 2 and 4) were immunoprecipitated by the anti–actinin-4 polyclonal antibody (lanes 1 and 2) or normal rabbit IgG (lanes 3 and 4). SDS-PAGE and autoradiography reveal that this polyclonal antibody is reactive with actinin-4 protein, but not with actinin-1. (C) Confocal immunofluorescence microscopy showing the subcellular localization of actinin-4 in lung fibroblasts MRC-5. Arrow indicates the nuclear staining, and arrowheads indicate linear staining of actinin-4 along actin stress fibers. Bar, 5 μm.
Figure 4
Reactivity of polyclonal antibody against actinin-4 peptide. (A) Immunoblot analysis reveals that the polyclonal antibody raised against actinin-4 peptide (lane 1) reacts with a single protein of ∼100 kD in the cell lysate of WiDr cells. A blot with normal rabbit IgG (lane 2) is included as a negative control. Molecular masses (in kD) are shown on the left. (B) In vitro translation products of actinin-4 (lanes 1 and 3) and actinin-1 (lanes 2 and 4) were immunoprecipitated by the anti–actinin-4 polyclonal antibody (lanes 1 and 2) or normal rabbit IgG (lanes 3 and 4). SDS-PAGE and autoradiography reveal that this polyclonal antibody is reactive with actinin-4 protein, but not with actinin-1. (C) Confocal immunofluorescence microscopy showing the subcellular localization of actinin-4 in lung fibroblasts MRC-5. Arrow indicates the nuclear staining, and arrowheads indicate linear staining of actinin-4 along actin stress fibers. Bar, 5 μm.
Figure 5
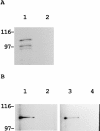
Actin-binding activity of actinin-4. (A) Direct association of actinin-4 with actin. In vitro translation product of actinin-4 was incubated with chicken gizzard actin-conjugated Sepharose 4B beads (lane 1) or Sepharose 4B beads alone (lane 2). After extensive washing, SDS-PAGE and autoradiography revealed that 35S-labeled actinin-4 protein was retained only in actin-coupled beads (lane 1). Molecular masses (in kD) are shown on the left. (B) Cell lysate of human squamous cell carcinoma cell line (PC-10) was incubated with actin-conjugated (lanes 1 and 3) or control (lanes 2 and 4) Sepharose 4B beads. Bound proteins were analyzed by immunoblotting. Approximately 100-kD proteins of actinin-1 (lane 1) and actinin-4 (lane 3) were detected with monoclonal antibodies BM-75.2 and NCC-Lu-632, respectively. Molecular masses (in kD) are shown on the left.
Figure 6
Confocal fluorescence microscopy showing actinins and the actin cytoskeleton in uterine endometrial fibroblasts. (A, C, and E) Double fluorescence of actinin-1 detected by BM-75.2 mAb (A and red in E) and actin by phalloidin-conjugated FITC (C and green in E) is shown. Actinin-1 is localized at the ends of actin stress fibers (E, arrowheads). (B, D, and F) Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (B and red in F) and actin by phalloidin-conjugated FITC (D and green in F) is shown. Actinin-4 is colocalized specifically with actin stress fibers (B, arrowheads). Bars, 5 μm.
Figure 7
Nuclear localization of actinin-4. (A) Confocal fluorescence microscopy showing subcellular localization of actinin-1 and the actin cytoskeleton in breast cancer cell line MCF7. Double fluorescence of actinin-1 detected by BM-75.2 mAb (red) and actin by phalloidin-conjugated FITC (green) is shown. Actinin-1 is concentrated specifically at focal adhesions and adherens junctions (arrowheads) (yellow), but not in the nucleus. (B) Confocal fluorescence microscopy showing nuclear localization of actinin-4 and the actin cytoskeleton in breast cancer cell line MCF7. Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (red) and actin by phalloidin-conjugated FITC (green) is shown. Actinin-4 is separated from the cytoplasmic actin cytoskeleton and localized specifically in the nucleus. (C) Immunoblot analysis of whole cell lysates from oral floor cancer cell line IMC2 (lane 1), urinary bladder cancer cell line KU7 (lane 2), and breast cancer cell lines MCF7 (lane 3) and R27 (lane 4) by mAb NCC-Lu-632 (left) and normal mouse IgM (negative control, right). IMC2, KU7, and MCF7 are cell lines showing the nuclear localization of actinin-4, and R27 is a cell line showing cytoplasmic localization. Molecular masses (in kD) are shown on the left. Bar, 5 μm.
Figure 7
Nuclear localization of actinin-4. (A) Confocal fluorescence microscopy showing subcellular localization of actinin-1 and the actin cytoskeleton in breast cancer cell line MCF7. Double fluorescence of actinin-1 detected by BM-75.2 mAb (red) and actin by phalloidin-conjugated FITC (green) is shown. Actinin-1 is concentrated specifically at focal adhesions and adherens junctions (arrowheads) (yellow), but not in the nucleus. (B) Confocal fluorescence microscopy showing nuclear localization of actinin-4 and the actin cytoskeleton in breast cancer cell line MCF7. Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (red) and actin by phalloidin-conjugated FITC (green) is shown. Actinin-4 is separated from the cytoplasmic actin cytoskeleton and localized specifically in the nucleus. (C) Immunoblot analysis of whole cell lysates from oral floor cancer cell line IMC2 (lane 1), urinary bladder cancer cell line KU7 (lane 2), and breast cancer cell lines MCF7 (lane 3) and R27 (lane 4) by mAb NCC-Lu-632 (left) and normal mouse IgM (negative control, right). IMC2, KU7, and MCF7 are cell lines showing the nuclear localization of actinin-4, and R27 is a cell line showing cytoplasmic localization. Molecular masses (in kD) are shown on the left. Bar, 5 μm.
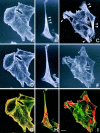
Figure 8
Confocal fluorescence microscopy showing the localization of actinins and the actin cytoskeleton in a colon cancer cell line SW480. (A, D, and G) Double fluorescence of actinin-1 detected by BM-75.2 mAb (A and red in G) and actin by phalloidin-conjugated FITC (D and green in G) is shown. Actinin-1 is colocalized with the cell membrane–associated cortical actin cytoskeleton. (B, E, and H) Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (B and red in H) and actin by phalloidin-conjugated FITC (E and green in H) is shown. Actinin-4 is concentrated in the sharply extended cytoplasm (B, arrowhead). (C, F, and I) Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (C and red in I) and actin by phalloidin-conjugated FITC (F and green in I) is shown. Cells at the edge of a cluster overexpress actinin-4 (C, arrowheads). Bar, 5 μm.
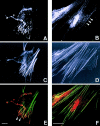
Figure 9
Wound assay for the detection of actinin-4 in cells forced to be motile. An artificial linear defect was introduced in confluent monolayers of A-431 cells. The expression of actinin-4 is shown by immunofluorescence microscopy. The cells along the edges of the wound (A) and the cells migrating into the wound (B–D) overexpress actinin-4. D is a higher-power view of B. Bars, 10 μm.
Figure 10
Immunofluorescence microscopy showing translocation of actinin-4 protein from the cytoplasm to the nucleus. (A) In control untreated HFK, actinin-4 was found to exist in the cytoplasm. (B) After treatment of HFK with wortmannin, actinin-4 protein was translocated into the nucleus. Bar, 5 μm.
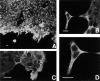
Figure 11
Immunohistochemical detection of actinin-4 in human tissues. Immunoperoxidase staining (ABC method) was performed on acetone-fixed paraffin-embedded (AMeX) (Sato et al., 1986) human tissues using NCC-Lu-632 mAb. (A) Normal mammary glands. Duct cells of normal mammary glands are stained at the epithelio–stromal and epithelio– myoepithelial borders, but the nuclei of normal mammary gland cells are not stained. (B) In papillotubular carcinoma of the breast, actinin-4 is localized in the nuclei (type A). (C) In scirrhous carcinoma of the breast, the cytoplasm of tumor cells is stained, but the nuclei of tumor cells are not (type C). (D) In invasive lobular carcinoma of the breast, the cytoplasm of the tumor cells is stained (type C). Bars, 30 μm.
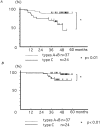
Figure 12
(A) Disease-free survival curves of 61 patients with clinical stages I and II breast cancer, according to the subcellular localization of actinin-4. Disease-free survival curves are drawn using the Kaplan-Meiler method. Significant difference in disease-free survival is observed between types A + B (nuclear) and type C (nonnuclear) (P < 0.01). (B) Overall survival curves of 61 patients with clinical stages I and II breast cancer, according to the subcellular localization of actinin-4. Significant difference in overall survival is observed between types A + B (nuclear) and type C (nonnuclear) (P < 0.01).
Similar articles
- Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion.
Nikolopoulos SN, Turner CE. Nikolopoulos SN, et al. J Cell Biol. 2000 Dec 25;151(7):1435-48. doi: 10.1083/jcb.151.7.1435. J Cell Biol. 2000. PMID: 11134073 Free PMC article. - [Novel splicing isoform of actin-binding protein alpha-actinin 4 in epidermoid carcinoma cells A431].
Aksenova VIu, Khotin MG, Turoverova LV, Iudintseva NM, Magnusson KÉ, Pinaev GP, Tentler DG. Aksenova VIu, et al. Tsitologiia. 2012;54(1):25-32. Tsitologiia. 2012. PMID: 22567897 Russian. - Mutant alpha-actinin-4 promotes tumorigenicity and regulates cell motility of a human lung carcinoma.
Menez J, Le Maux Chansac B, Dorothée G, Vergnon I, Jalil A, Carlier MF, Chouaib S, Mami-Chouaib F. Menez J, et al. Oncogene. 2004 Apr 8;23(15):2630-9. doi: 10.1038/sj.onc.1207347. Oncogene. 2004. PMID: 15048094 - Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton.
Jiang P, Enomoto A, Takahashi M. Jiang P, et al. Cancer Lett. 2009 Nov 1;284(2):122-30. doi: 10.1016/j.canlet.2009.02.034. Epub 2009 Mar 19. Cancer Lett. 2009. PMID: 19303207 Review. - Fascin1 in carcinomas: Its regulation and prognostic value.
Ma Y, Machesky LM. Ma Y, et al. Int J Cancer. 2015 Dec 1;137(11):2534-44. doi: 10.1002/ijc.29260. Epub 2014 Oct 28. Int J Cancer. 2015. PMID: 25302416 Review.
Cited by
- Postmitotic expansion of cell nuclei requires nuclear actin filament bundling by α-actinin 4.
Krippner S, Winkelmeier J, Knerr J, Brandt DT, Virant D, Schwan C, Endesfelder U, Grosse R. Krippner S, et al. EMBO Rep. 2020 Nov 5;21(11):e50758. doi: 10.15252/embr.202050758. Epub 2020 Sep 22. EMBO Rep. 2020. PMID: 32959960 Free PMC article. - A retrovirus-based protein complementation assay screen reveals functional AKT1-binding partners.
Ding Z, Liang J, Lu Y, Yu Q, Songyang Z, Lin SY, Mills GB. Ding Z, et al. Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15014-9. doi: 10.1073/pnas.0606917103. Epub 2006 Oct 3. Proc Natl Acad Sci U S A. 2006. PMID: 17018644 Free PMC article. - Motility-related proteins as markers for head and neck squamous cell cancer.
Abraham MT, Kuriakose MA, Sacks PG, Yee H, Chiriboga L, Bearer EL, Delacure MD. Abraham MT, et al. Laryngoscope. 2001 Jul;111(7):1285-9. doi: 10.1097/00005537-200107000-00027. Laryngoscope. 2001. PMID: 11568556 Free PMC article. - Alpha-actinin 4 and tumorigenesis of hepatocellular carcinoma.
Wang G, Li Y, Tang B, Yu Q. Wang G, et al. Transl Cancer Res. 2019 Aug;8(4):1374-1380. doi: 10.21037/tcr.2019.07.34. Transl Cancer Res. 2019. PMID: 35116880 Free PMC article. - WAVE2 is associated with poor prognosis in pancreatic cancers and promotes cell motility and invasiveness via binding to ACTN4.
Taniuchi K, Furihata M, Naganuma S, Saibara T. Taniuchi K, et al. Cancer Med. 2018 Nov;7(11):5733-5751. doi: 10.1002/cam4.1837. Epub 2018 Oct 23. Cancer Med. 2018. PMID: 30353690 Free PMC article.
References
- Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. - PubMed
- Bao L, Loda M, Janmey AP, Stewart R, Anand-Apte B, Zetter BR. Tymosin β15: a novel regulator of tumor cell motility upregulated in metastatic prostate cancer. Nat Med. 1996;2:1322–1328. - PubMed
- Beggs AH, Byers TJ, Knoll JHM, Boyce FM, Bruns GAP, Kunkel LM. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. - PubMed
- Boulikas T. Nuclear localization signals (NLS) Crit Rev Eukaryotic Gene Expr. 1993;3:193–227. - PubMed
- Burridge K, Nuckolls G, Otey C, Pavalko F, Simon K, Turner C. Actin-membrane interaction in focal adhesions. Cell Differ Dev. 1990;32:337–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous