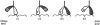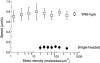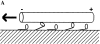Processivity of the motor protein kinesin requires two heads - PubMed (original) (raw)
Processivity of the motor protein kinesin requires two heads
W O Hancock et al. J Cell Biol. 1998.
Abstract
A single kinesin molecule can move for hundreds of steps along a microtubule without dissociating. One hypothesis to account for this processive movement is that the binding of kinesin's two heads is coordinated so that at least one head is always bound to the microtubule. To test this hypothesis, the motility of a full-length single-headed kinesin heterodimer was examined in the in vitro microtubule gliding assay. As the surface density of single-headed kinesin was lowered, there was a steep fall both in the rate at which microtubules landed and moved over the surface, and in the distance that microtubules moved, indicating that individual single-headed kinesin motors are not processive and that some four to six single-headed kinesin molecules are necessary and sufficient to move a microtubule continuously. At high ATP concentration, individual single-headed kinesin molecules detached from microtubules very slowly (at a rate less than one per second), 100-fold slower than the detachment during two-headed motility. This slow detachment directly supports a coordinated, hand-over-hand model in which the rapid detachment of one head in the dimer is contingent on the binding of the second head.
Figures
Figure 1
The hand-over-hand model. It is postulated that the unbinding of the kinesin's first head (unshaded) in step iv occurs only after the binding of the second head (shaded) in step iii. In this way at least one head remains bound to the microtubule at all times. Also shown are the likely nucleotide states of the two heads during the motion (see text). The results presented in this paper indicate that the structure with both heads attached (iii) is highly strained and that this strain accelerates the release of the trailing head.
Figure 2
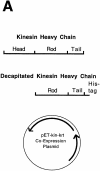
Protocol for generating single-headed kinesin heterodimers. (A) Coexpression vector. (B) Protein expression and purification strategy in which the single-headed heterodimers were separated from the two-headed homodimers by chromatography and centrifugation.
Figure 2
Protocol for generating single-headed kinesin heterodimers. (A) Coexpression vector. (B) Protein expression and purification strategy in which the single-headed heterodimers were separated from the two-headed homodimers by chromatography and centrifugation.
Figure 3
SDS-PAGE of bacterial supernatant and Ni column fractions. Lane 1, molecular weight markers; lane 2, bacterial lysate supernatant loaded onto the Ni column (diluted 1:4); lanes 3–5, column flow-through fractions (diluted 1:4), lane 6: 50 μg/ml BSA standard; lanes 7–12, elution fractions.
Figure 4
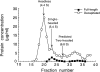
Separation of kinesin species by sucrose density–gradient centrifugation. The concentrations of full-length kinesin heavy chain and decapitated kinesin heavy chain peptides in each fraction were estimated from scans of Coomassie blue–stained SDS-PAGE gels. Inferred positions of the peak headless and single-headed fractions are highlighted. There was no evidence for a two-headed kinesin peak; the expected position of the two-headed peak is based on separate centrifugation experiments with pure wild-type kinesin. Fractions 22–24 were used as single-headed kinesin samples for motility assays.
Figure 5
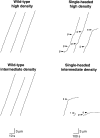
Displacement versus time traces for microtubules moving over surfaces coated with wild-type kinesin (left panels) and single-headed kinesin (right panels) at high motor density (280 motors/μm2; top panels) and intermediate motor density (60 motors/μm2, bottom panels). The tracks of 12 microtubules are shown. The position of the leading end of each microtubule was measured every second for wild-type kinesin or every 10 s for single-headed kinesin. Note that microtubules often paused (p) or stalled (s) when moving over surfaces coated with single-headed kinesin. The short trace in the bottom right panel corresponds to a microtubule that released from the surface and diffused away (r). Note that the scale bars correspond to 10 s for wild-type kinesin and 100 s for single-headed kinesin, so the similar slopes of the traces indicates that wild-type kinesin moves about ten times faster than single-headed kinesin.
Figure 6
Microtubule gliding speeds for wild-type kinesin (open squares) and single-headed kinesin (closed circles). Each point is the average of 7–18 observations. Error bars are standard deviations. For single-headed kinesin, speeds were determined during windows of smooth movement.
Figure 7
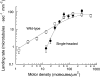
Landing rate profile for wild-type (open squares) and single-headed kinesin (closed circles). The landing rate was measured by counting the number of microtubules that landed and moved ⩾0.3 μm in a 4000-μm2 video screen. Each data point is derived from two to six video screens taken from each of one or two flow cells. Standard error bars were calculated according to Materials and Methods. The continuous curves are the fits to the model of Equation 2 with n = 1 (wild-type) and n = 5 (single-headed).
Figure 8
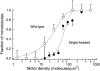
The fraction of microtubules that moved a distance greater than their length as a function of motor density. Microtubules of length between two and three micrometers were observed for 30 s for wild-type kinesin (open squares) or for 300 s for single-headed kinesin (closed symbols). Standard error bars were calculated according to Materials and Methods. The continuous curves are the fits to the model of Eq. 3 with n = 1 (wild-type) and n = 4 (single-headed).
Figure 9
The fraction of attached microtubules that moved ⩾0.3 μm as a function of motor density. Only microtubules of length 1 μm or longer that landed on the surface for 2 s were counted. The bars represent standard errors of the mean. (B): The duration of binding events for microtubules that landed on a surface coated with a low density of single-headed kinesin and swiveled, indicating single motor interactions. Although every event of duration 2 s or longer was counted, the count for 1-s events is likely to be an underestimate due to missed events. A fit of the data to a single exponential curve with a time constant of 3.1 s is shown. The ATP concentration was 1 mM.
Figure 9
The fraction of attached microtubules that moved ⩾0.3 μm as a function of motor density. Only microtubules of length 1 μm or longer that landed on the surface for 2 s were counted. The bars represent standard errors of the mean. (B): The duration of binding events for microtubules that landed on a surface coated with a low density of single-headed kinesin and swiveled, indicating single motor interactions. Although every event of duration 2 s or longer was counted, the count for 1-s events is likely to be an underestimate due to missed events. A fit of the data to a single exponential curve with a time constant of 3.1 s is shown. The ATP concentration was 1 mM.
Figure 10
(A) Coordination between single-headed molecules moving a microtubule. In this example, the binding of the fourth head and its subsequent conformational change is hypothesized to accelerate the unbinding of the second, attached head and to move the microtubule a small distance to a position that will allow one of the other detached heads to bind. (B) Model of organelle transport by monomeric KIF1 molecules. If these kinesin-related proteins are coordinated like single-headed kinesin, then continuous motility would require only four to six molecules on the surface of a mitochondrion.
Figure 10
(A) Coordination between single-headed molecules moving a microtubule. In this example, the binding of the fourth head and its subsequent conformational change is hypothesized to accelerate the unbinding of the second, attached head and to move the microtubule a small distance to a position that will allow one of the other detached heads to bind. (B) Model of organelle transport by monomeric KIF1 molecules. If these kinesin-related proteins are coordinated like single-headed kinesin, then continuous motility would require only four to six molecules on the surface of a mitochondrion.
Similar articles
- Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains.
Hancock WO, Howard J. Hancock WO, et al. Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13147-52. doi: 10.1073/pnas.96.23.13147. Proc Natl Acad Sci U S A. 1999. PMID: 10557288 Free PMC article. - Role of the kinesin neck region in processive microtubule-based motility.
Romberg L, Pierce DW, Vale RD. Romberg L, et al. J Cell Biol. 1998 Mar 23;140(6):1407-16. doi: 10.1083/jcb.140.6.1407. J Cell Biol. 1998. PMID: 9508773 Free PMC article. - Direct observation of single kinesin molecules moving along microtubules.
Vale RD, Funatsu T, Pierce DW, Romberg L, Harada Y, Yanagida T. Vale RD, et al. Nature. 1996 Apr 4;380(6573):451-3. doi: 10.1038/380451a0. Nature. 1996. PMID: 8602245 Free PMC article. - Directionality of kinesin motors.
Kasprzak AA, Hajdo Ł. Kasprzak AA, et al. Acta Biochim Pol. 2002;49(4):813-21. Acta Biochim Pol. 2002. PMID: 12545188 Review. - Move in for the kill: motile microtubule regulators.
Su X, Ohi R, Pellman D. Su X, et al. Trends Cell Biol. 2012 Nov;22(11):567-75. doi: 10.1016/j.tcb.2012.08.003. Epub 2012 Sep 6. Trends Cell Biol. 2012. PMID: 22959403 Free PMC article. Review.
Cited by
- The E-hook of tubulin interacts with kinesin's head to increase processivity and speed.
Lakämper S, Meyhöfer E. Lakämper S, et al. Biophys J. 2005 Nov;89(5):3223-34. doi: 10.1529/biophysj.104.057505. Epub 2005 Aug 12. Biophys J. 2005. PMID: 16100283 Free PMC article. - Kinesin motor mechanics: binding, stepping, tracking, gating, and limping.
Block SM. Block SM. Biophys J. 2007 May 1;92(9):2986-95. doi: 10.1529/biophysj.106.100677. Epub 2007 Feb 26. Biophys J. 2007. PMID: 17325011 Free PMC article. Review. No abstract available. - A mechanistic model for the organization of microtubule asters by motor and non-motor proteins in a mammalian mitotic extract.
Chakravarty A, Howard L, Compton DA. Chakravarty A, et al. Mol Biol Cell. 2004 May;15(5):2116-32. doi: 10.1091/mbc.e03-08-0579. Epub 2004 Feb 20. Mol Biol Cell. 2004. PMID: 14978218 Free PMC article. - Kinesin processivity.
Taylor EW, Borisy GG. Taylor EW, et al. J Cell Biol. 2000 Nov 27;151(5):F27-9. doi: 10.1083/jcb.151.5.f27. J Cell Biol. 2000. PMID: 11086015 Free PMC article. Review. No abstract available. - Dynein-mediated cargo transport in vivo. A switch controls travel distance.
Gross SP, Welte MA, Block SM, Wieschaus EF. Gross SP, et al. J Cell Biol. 2000 Mar 6;148(5):945-56. doi: 10.1083/jcb.148.5.945. J Cell Biol. 2000. PMID: 10704445 Free PMC article.
References
- Berliner E, Young EC, Anderson K, Mahtani HK, Gelles J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature. 1995;373:718–721. - PubMed
- Block SM, Goldstein LS, Schnapp BJ. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. - PubMed
- Bloom GS, Endow SA. Motor proteins 1: kinesins. Protein Profile. 1995;2:1105–1171. - PubMed
- Bloom GS, Wagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27:3409–3416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials