The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling - PubMed (original) (raw)
The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling
M Mancini et al. J Cell Biol. 1998.
Abstract
Caspase-3-mediated proteolysis is a critical element of the apoptotic process. Recent studies have demonstrated a central role for mitochondrial proteins (e.g., Bcl-2 and cytochrome c) in the activation of caspase-3, by a process that involves interaction of several protein molecules. Using antibodies that specifically recognize the precursor form of caspase-3, we demonstrate that the caspase-3 proenzyme has a mitochondrial and cytosolic distribution in nonapoptotic cells. The mitochondrial caspase-3 precursor is contained in the intermembrane space. Delivery of a variety of apoptotic stimuli is accompanied by loss of mitochondrial caspase-3 precursor staining and appearance of caspase-3 proteolytic activity. We propose that the mitochondrial subpopulation of caspase-3 precursor molecules is coupled to a distinct subset of apoptotic signaling pathways that are Bcl-2 sensitive and that are transduced through multiple mitochondrion-specific protein interactions.
Figures
Figure 1
Characterization of anti–caspase-3 antiserum (R280). (a) 10-ng amounts of the purified large subunits (caspases 1–10, excluding caspase-6) and Ced-3 were immunoblotted with R280 antiserum. (b) Biotinylated recombinant mature caspase-3 was denatured by treatment with guanidium-HCl or SDS, as described in the Materials and Methods section. Native and denatured biotinylated caspase-3 were subsequently immunoprecipitated using R280, and the p17 subunit was detected by immunoblotting. (c) 46 μg of HUVEC lysate was immunoblotted with affinity-purified R280 antiserum. Similar results were obtained when keratinocyte lysates were immunoblotted with affinity-purified R280 antiserum (data not shown).
Figure 3
Biochemical analysis of mitochondrial caspase-3 precursor. (a) Caspase-3 precursor is detected by immunoblotting in human liver mitochondria. 200-μg aliquots of mitochondria, prepared from human liver as described in Materials and Methods, were incubated for 1 h at 37°C in the presence of the following: no additions (lane 1), 0.2 μg granzyme B (lane 2), 1% NP-40 (lane 3), or 1% NP-40 and 0.2 μg granzyme B (lane 4). Equal aliquots of these samples were electrophoresed and immunoblotted with the R280 anti–caspase-3 antibody. Note that the R280 antibody does not blot the 17-kD active enzyme well; the exposure shown in the figure was chosen to optimize visualization of the decreased 32-kD precursor caspase-3 (lane 4). The results shown are representative of three separate experiments. (b) Mitochondrial pro-caspase-3 colocalizes with adenylate kinase in the intermembrane space. Freshly isolated rat liver mitochondria were sub-fractionated by progressive digitonin treatment as described in Materials and Methods. Marker enzyme activities for the intermembrane space (adenylate kinase; circles) and matrix (fumarase; triangles) were measured in the resulting supernatants (solid symbols) and pellets (open symbols). Pro-caspase-3 (squares) was detected in the same fractions by immunoblotting using the R280 antibody that cross-reacts with rat pro-caspase-3. Marker enzyme and pro-caspase-3 distributions in supernatants and pellets are expressed as a percentage of the total recovered. (c) Distribution of caspase-3 precursor in HeLa cells. HeLa cells were fractionated into cytosolic (C) and mitochondrial (M) fractions as described in Materials and Methods. Equivalent volume amounts of cytosolic and mitochondrial fractions were electrophoresed, and relative amounts of caspase-3 precursor, cytochrome c, and cytochrome oxidase (subunit IV) were determined by immunoblotting. Results are representative of two separate experiments.
Figure 3
Biochemical analysis of mitochondrial caspase-3 precursor. (a) Caspase-3 precursor is detected by immunoblotting in human liver mitochondria. 200-μg aliquots of mitochondria, prepared from human liver as described in Materials and Methods, were incubated for 1 h at 37°C in the presence of the following: no additions (lane 1), 0.2 μg granzyme B (lane 2), 1% NP-40 (lane 3), or 1% NP-40 and 0.2 μg granzyme B (lane 4). Equal aliquots of these samples were electrophoresed and immunoblotted with the R280 anti–caspase-3 antibody. Note that the R280 antibody does not blot the 17-kD active enzyme well; the exposure shown in the figure was chosen to optimize visualization of the decreased 32-kD precursor caspase-3 (lane 4). The results shown are representative of three separate experiments. (b) Mitochondrial pro-caspase-3 colocalizes with adenylate kinase in the intermembrane space. Freshly isolated rat liver mitochondria were sub-fractionated by progressive digitonin treatment as described in Materials and Methods. Marker enzyme activities for the intermembrane space (adenylate kinase; circles) and matrix (fumarase; triangles) were measured in the resulting supernatants (solid symbols) and pellets (open symbols). Pro-caspase-3 (squares) was detected in the same fractions by immunoblotting using the R280 antibody that cross-reacts with rat pro-caspase-3. Marker enzyme and pro-caspase-3 distributions in supernatants and pellets are expressed as a percentage of the total recovered. (c) Distribution of caspase-3 precursor in HeLa cells. HeLa cells were fractionated into cytosolic (C) and mitochondrial (M) fractions as described in Materials and Methods. Equivalent volume amounts of cytosolic and mitochondrial fractions were electrophoresed, and relative amounts of caspase-3 precursor, cytochrome c, and cytochrome oxidase (subunit IV) were determined by immunoblotting. Results are representative of two separate experiments.
Figure 3
Biochemical analysis of mitochondrial caspase-3 precursor. (a) Caspase-3 precursor is detected by immunoblotting in human liver mitochondria. 200-μg aliquots of mitochondria, prepared from human liver as described in Materials and Methods, were incubated for 1 h at 37°C in the presence of the following: no additions (lane 1), 0.2 μg granzyme B (lane 2), 1% NP-40 (lane 3), or 1% NP-40 and 0.2 μg granzyme B (lane 4). Equal aliquots of these samples were electrophoresed and immunoblotted with the R280 anti–caspase-3 antibody. Note that the R280 antibody does not blot the 17-kD active enzyme well; the exposure shown in the figure was chosen to optimize visualization of the decreased 32-kD precursor caspase-3 (lane 4). The results shown are representative of three separate experiments. (b) Mitochondrial pro-caspase-3 colocalizes with adenylate kinase in the intermembrane space. Freshly isolated rat liver mitochondria were sub-fractionated by progressive digitonin treatment as described in Materials and Methods. Marker enzyme activities for the intermembrane space (adenylate kinase; circles) and matrix (fumarase; triangles) were measured in the resulting supernatants (solid symbols) and pellets (open symbols). Pro-caspase-3 (squares) was detected in the same fractions by immunoblotting using the R280 antibody that cross-reacts with rat pro-caspase-3. Marker enzyme and pro-caspase-3 distributions in supernatants and pellets are expressed as a percentage of the total recovered. (c) Distribution of caspase-3 precursor in HeLa cells. HeLa cells were fractionated into cytosolic (C) and mitochondrial (M) fractions as described in Materials and Methods. Equivalent volume amounts of cytosolic and mitochondrial fractions were electrophoresed, and relative amounts of caspase-3 precursor, cytochrome c, and cytochrome oxidase (subunit IV) were determined by immunoblotting. Results are representative of two separate experiments.
Figure 7
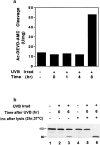
Time course of appearance of cleavage activity in keratinocyte lysates after UVB irradiation. (a) Whole cell lysates prepared at various times after UVB irradiation were assayed for cleavage of the fluorogenic substrate Ac-DEVD-AMC as described in the Materials and Methods section. Cleavage activity is expressed in U/mg, where a unit is defined as the amount of enzyme needed to produce 1 pmol per min using 100 μM Ac-DEVD-AMC. Four separate experiments yielded similar results. (b) PARP cleavage is observed in keratinocytes 6 h after UV irradiation. Irradiated and control nonirradiated keratinocytes were lysed at the indicated times as described in Materials and Methods. Whole cell lysates (lanes 1–3), as well as whole cell lysates incubated in vitro for 2 h at 37°C (lanes 4–6), were immunoblotted with a serum recognizing PARP (Casciola-Rosen et al., 1995). The migration positions of intact PARP (113 kD) and its cleavage fragment (89 kD) are marked. 60 μg of protein was loaded in each lane. This experiment was repeated on three separate occasions with similar results. Identical results were obtained using HeLa cells.
Figure 2
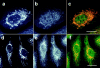
Caspase-3 is localized in the cytosol and mitochondria of keratinocytes and HUVECs. Keratinocytes (a–c) and HUVECs (d–f) were labeled with MitoTracker, fixed, permeabilized, and stained with affinity-purified polyclonal rabbit anti–caspase-3 precursor antibody (R280) as described in Materials and Methods. The stained cells were examined by confocal immunofluorescence microscopy. Caspase-3 antibodies were visualized with fluorescein-conjugated goat anti–rabbit IgG and assigned the color green (b and e), whereas mitochondria labeled with MitoTracker were assigned the color red (a and d). When red and green images were merged, overlapping red and green pixels appeared orange/yellow (c and f). The experiments were repeated on 11 (a–c) or 4 (d–f) separate occasions with identical results. Bar, 10 μm.
Figure 4
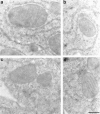
Immunolocalization of the caspase-3 precursor. Ultrathin sections of cultured WIF-B cells were labeled with affinity-purified R280 antibodies and 12-nm colloidal gold conjugated to donkey anti–rabbit IgG. a–d show caspase-3 precursor staining within mitochondria. Gold particles were localized to just inside the outer membrane (a and c), as well as to inner membrane cristae (a, b, and d). Omission of primary antibody resulted in the complete abolition of both cytosolic and mitochondrial staining (data not shown). Similar results were obtained in HeLa cells (data not shown). Bar, 300 nm.
Figure 5
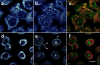
Lack of mitochondrial caspase-3 precursor staining in keratinocytes induced to become apoptotic by UVB irradiation. Control nonirradiated keratinocytes (a–c) or keratinocytes UVB-irradiated and subsequently incubated for 8 h (d–f) were labeled with MitoTracker and then fixed, permeabilized, and stained with R280. Mitochondrial staining was visualized in red (a and d), whereas pro-caspase-3 staining was visualized in green (b and e). When images were merged (c and f), overlapping red and green pixels appeared orange/yellow. Arrowheads denote apoptotic surface blebs. Experiments were repeated on 10 separate occasions with similar results. Bar, 10 μm.
Figure 6
Lack of mitochondrial caspase-3 precursor staining and MitoTracker labeling in staurosporine-induced apoptosis. Keratinocytes were incubated with 5 μM staurosporine for 4.5 h before MitoTracker labeling (c) and double-staining with DAPI (a) and affinity-purified R280 antibodies (b). (a) DAPI staining of nuclei. Arrowheads denote fragmented, condensed nuclei typical of apoptotic cells; the arrow marks a normal, nonapoptotic nucleus. (b) R280 staining of pro-caspase-3. Caspase-3 precursor stains in normal cells (arrow) but is absent in apoptotic cells (arrowhead). (c) MitoTracker staining of mitochondria. Mitochondria are labeled with MitoTracker in cells with a normal nucleus (arrow) but do not label in cells with apoptotic nuclei (arrowhead). (a–c) These results are representative of three separate experiments. Bar, 10 μm.
Similar articles
- Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3.
Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K. Amarante-Mendes GP, et al. Blood. 1998 Mar 1;91(5):1700-5. Blood. 1998. PMID: 9473236 - Detection of pro-caspase-3 in cytosol and mitochondria of various tissues.
Samali A, Zhivotovsky B, Jones DP, Orrenius S. Samali A, et al. FEBS Lett. 1998 Jul 17;431(2):167-9. doi: 10.1016/s0014-5793(98)00740-6. FEBS Lett. 1998. PMID: 9708895 - IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases.
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. Deveraux QL, et al. EMBO J. 1998 Apr 15;17(8):2215-23. doi: 10.1093/emboj/17.8.2215. EMBO J. 1998. PMID: 9545235 Free PMC article. - Cytochrome c in the apoptotic and antioxidant cascades.
Skulachev VP. Skulachev VP. FEBS Lett. 1998 Feb 27;423(3):275-80. doi: 10.1016/s0014-5793(98)00061-1. FEBS Lett. 1998. PMID: 9515723 Review. - The central executioners of apoptosis: caspases or mitochondria?
Green D, Kroemer G. Green D, et al. Trends Cell Biol. 1998 Jul;8(7):267-71. doi: 10.1016/s0962-8924(98)01273-2. Trends Cell Biol. 1998. PMID: 9714597 Review.
Cited by
- Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines.
Çiftçi GA, Işcan A, Kutlu M. Çiftçi GA, et al. Cytotechnology. 2015 Oct;67(5):893-904. doi: 10.1007/s10616-015-9877-6. Epub 2015 Apr 24. Cytotechnology. 2015. PMID: 25906387 Free PMC article. - S-Nitrosylation of mitochondrial caspases.
Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, Gaston B. Mannick JB, et al. J Cell Biol. 2001 Sep 17;154(6):1111-6. doi: 10.1083/jcb.200104008. Epub 2001 Sep 10. J Cell Biol. 2001. PMID: 11551979 Free PMC article. - Mechanisms of apoptosis.
Reed JC. Reed JC. Am J Pathol. 2000 Nov;157(5):1415-30. doi: 10.1016/S0002-9440(10)64779-7. Am J Pathol. 2000. PMID: 11073801 Free PMC article. - Evidence for redox regulation of cytochrome C release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition.
Kirkland RA, Franklin JL. Kirkland RA, et al. J Neurosci. 2001 Mar 15;21(6):1949-63. doi: 10.1523/JNEUROSCI.21-06-01949.2001. J Neurosci. 2001. PMID: 11245680 Free PMC article. - Transition from caspase-dependent to caspase-independent mechanisms at the onset of apoptotic execution.
Samejima K, Toné S, Kottke TJ, Enari M, Sakahira H, Cooke CA, Durrieu F, Martins LM, Nagata S, Kaufmann SH, Earnshaw WC. Samejima K, et al. J Cell Biol. 1998 Oct 5;143(1):225-39. doi: 10.1083/jcb.143.1.225. J Cell Biol. 1998. PMID: 9763434 Free PMC article.
References
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan JY. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
- Balch WE, Rothman JE. Characterization of protein transport between successive compartments of the Golgi: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985;240:413–425. - PubMed
- Berryman MA, Porter WR, Rodewald RD, Hubbard AL. Effects of tannic acid on antigenicity and membrane contrast in ultrastructural immunocytochemistry. J Histochem Cytochem. 1992;40:845–857. - PubMed
- Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/ APO-1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. - PubMed
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR044684/AR/NIAMS NIH HHS/United States
- K12DK01298/DK/NIDDK NIH HHS/United States
- T32 AI007247/AI/NIAID NIH HHS/United States
- 5T32-AI07247/AI/NIAID NIH HHS/United States
- AR44684/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous