PcaU, a transcriptional activator of genes for protocatechuate utilization in Acinetobacter - PubMed (original) (raw)
PcaU, a transcriptional activator of genes for protocatechuate utilization in Acinetobacter
U Gerischer et al. J Bacteriol. 1998 Mar.
Abstract
The Acinetobacter pcaIJFBDKCHG operon encodes the six enzymes that convert protocatechuate to citric acid cycle intermediates. Directly downstream from the operon are qui and pob genes encoding sets of enzymes that convert quinate and p-hydroxybenzoate, respectively, to protocatechuate. Prior to this investigation, the only known regulatory gene in the pca-qui-pob cluster was pobR, which encodes a transcriptional activator that responds to p-hydroxybenzoate and activates transcription of pobA. The pca and qui genes were known to be expressed in response to protocatechuate, but a protein that mediated this induction had not been identified. This study was initiated by characterization of a spontaneous mutation that mapped upstream from pcaI and prevented expression of the pca genes. Sequencing of wild-type DNA extending from the translational start of pcaI through and beyond the location of the mutation revealed a 282-bp intergenic region and a divergently transcribed open reading frame, designated pcaU. Downstream from pcaU are two open reading frames encoding proteins similar in amino acid sequence to those associated with the oxidation of acyl thioesters. Inactivation of pcaU reduced the induced expression of pca structural genes by about 90% and impeded but did not completely prevent growth of the mutant cells with protocatechuate. PcaU was expressed in Escherichia coli and shown to bind to a portion of the pcaI-pcaU intergenic region containing a sequence identical in 16 of 19 nucleotide residues to a segment of the pob operator. Further similarity of the two regulatory systems is indicated by 54% amino acid sequence identity in the aligned primary structures of PobR and PcaU. The pob and pca systems were shown to differ, however, in the relative orientations of transcriptional starts with respect to the site where the activator binds to DNA, the size of the intergenic region, and the tightness of transcriptional control. The spontaneous mutation blocking pca gene expression was located in the promoter for the pca operon. The 19-nucleotide residue operator sequences were shown to be parts of a consensus associated with transcriptional activation of genes associated with protocatechuate catabolism. Two different binding sites for Pseudomonas putida PcaR differ from the consensus in only a single nucleotide residue, and DNA directly downstream from Acinetobacter pcaU contains a 19-bp segment differing from the consensus in only two residues. PcaU was shown to bind to DNA containing this segment as well as to the DNA in the pcaU-pcaI intergenic region.
Figures
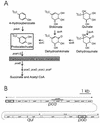
FIG. 1
Organization of genes associated with protocatechuate catabolism in Acinetobacter. (A) Various growth substrates are catabolized through pathways converging upon protocatechuate (open box). In Acinetobacter, protocatechuate elicits expression of all of the depicted genes except pobA, which is expressed in response to 4-hydroxybenzoate. Protocatechuate is converted to succinate and acetyl CoA by the consecutive actions of enzymes encoded by the pca genes. Null mutations in pcaB cause accumulation of the toxic metabolite carboxymuconate from protocatechuate; exposure of cells containing the Δ_pcaBDK1_ deletion to growth media supplemented with protocatechuate selects colonies that fail to express pcaH and -G. Most of these colonies are double mutants containing Δ_pcaBDK1_ and a mutation in pcaH or -G (21). In this paper, we describe another mutation, pcaP1, that blocks the promoter for the pca operon. Characterization of DNA flanking this mutation revealed pcaU. (B) Genes required for catabolism of the compounds shown in panel A are grouped within the 20 kb of contiguous DNA forming the pca-qui-pob cluster in the Acinetobacter chromosome. Directions of transcription are indicated by dashed arrows, and the position of Δ_pcaBDK1_ is shown by the grey rectangle. As described in this communication, expression of the pca genes in response to protocatechuate is governed by the divergently transcribed activator encoded by pcaU (dark border). The pobA gene (10) encodes 4-hydroxybenzoate hydroxylase and is regulated independently of the pca and qui genes by the divergently transcribed pobR (dark border) (9, 11).
FIG. 2
Restriction fragments used to characterize mutations blocking expression of pcaH and -G. Open rectangles represent the pca genes drawn to a scale proportionate to their length; the black rectangle represents the pcaU-pcaI intergenic region. Dashed horizontal arrows indicate the directions of transcription. Vertical arrows above the genes indicate the locations of mutations blocking expression of pcaH and -G, the structural genes for protocatechuate oxygenase (Fig. 1). Large shaded rectangles indicate the inserts within each recombinant plasmid; the name of the corresponding parental plasmid is enclosed in parentheses. Strains containing both Δ_pcaBDK1_ and null pcaH or -G mutations can be restored to wild type by transformation with the insert from pZR1. Strains containing null pcaH or -G mutations can be restored to wild type by transformation with the insert from pZR2. Strain ADP5126(Δ_pcaB′DK′1_, Δ_catD101_::Kmr pcaP1) is unusual in that it does not recover wild-type pca functions when it is transformed with pZR1 (21). Restoration of wild-type pcaD to this strain by transformation with pZR3 yielded a recombinant [strain ADP6126(Δ_catD101_::Kmr pcaP1)] that grew extremely slowly with _p_-hydroxybenzoate; the organism’s inability to metabolize protocatechuate was further indicated by the accumulation of the compound in the growth medium. Enzymatic analysis showed that pca genes are not expressed constitutively in strain ADP6126. The pca function missing in ADP6126 was not supplied by pZR2 but, as described in this paper, was provided by pZR9. The _Sau_3A restriction fragment in pZR9 contains a portion of pcaU, the pcaU-pcaI intergenic region, and pca structural genes extending from pcaI to the _Sau_3A site within pcaD.
FIG. 3
Recombinant plasmids containing all or part of pcaU. Symbols and markings are the same as described for Fig. 2.
FIG. 4
Similar deduced amino acid sequences of Acinetobacter PcaU and PobR. The aligned amino acid sequences are flanked by their encoding nucleotide sequences. Asterisks mark identical residues in the aligned amino acid sequences; nucleotide sequences extending both upstream and downstream from pcaU are shown. Two methionine codons, separated by nine nucleotide residues, are plausible start codons for pcaU translation; these codons are underlined. Because of uncertainty about the start codons, it is possible to conclude only that PcaU contains either 274 or 278 residues. The weight matrix comparison method of Dodd and Egan (12) assessed the likelihood of the shaded PcaU sequence being a helix-turn-helix DNA binding motif to be 100%, on the basis of a standard deviation score of 4.6; the corresponding value for the shaded PobR sequence is 5.2 (9). Restriction sites used in this investigation are labelled and marked by double overlining. Dashed arrows both upstream and downstream of pcaU indicate inverted repetitions that subsequent experiments demonstrated to be in sites where PcaU binds to DNA. Double overlining and underlining mark the _Eco_RI and _Xmn_I sites bordering an upstream DNA fragment that was shown to bind PcaU. Double-dashed arrows indicate the positions of primers used to amplify a downstream DNA fragment that binds PcaU.
FIG. 5
Functions of nucleotide sequences downstream and upstream from pcaU. (A) Two open reading frames, designated orf1 and orf2, encode amino acid sequences closely resembling those of enzymes associated with β-oxidation of fatty acids. Dashed arrows below gene designations show directions of transcription. The other dashed arrows indicate positions of inverted repetitions that were depicted in Fig. 4 and shown in later experiments to be within sites where PcaU binds to DNA. To provide a frame of reference for subsequent observations, the position of the nucleotide substitution causing the pcaP1 mutation also is shown here, as is the _Eco_RI site between pcaU and pcaI. (B) Alignment of nucleotide sequences containing inverted repetitions downstream and upstream from pcaU. Identical residues in the aligned sequences are in shaded boxes.
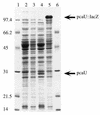
FIG. 6
Expression of pcaU in E. coli. Denaturing sodium dodecyl sulfate-gel (12.5% [wt/vol] acrylamide) electrophoresis of crude extracts (100 μg of protein per lane) of E. coli DH5α strains containing the following: lanes 1 and 6, molecular weight standards; lane 2, the unmodified vector pWH661+; lane 3, pZR18 containing pcaU oriented so that its transcription is not under the control of lacP; lane 4, pZR22 containing pcaU oriented so that its transcription is initiated by lacP; lane 5, pZR26 (pZR22 modified by insertion of a _lacZ_-Knr cassette into pcaU).
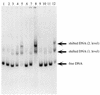
FIG. 7
Binding of PcaU to a DNA fragment containing a portion of the pcaU-pcaI intergenic region. The probe for this gel shift experiment was the 216-bp _Xmn_I-_Eco_RI fragment containing a portion of pcaU (Fig. 4). Lane 1 depicts migration of the probe without added extract. Incubation of the probe with 15 μg of protein from E. coli DH5α(pWH661+) (lane 2) or E. coli DH5α(pZR26) (lane 6) did not shift the migration pattern, whereas incubation of the probe with the same amount of protein from E. coli DH5α(pZR22) gave rise to two bands (lanes 5 and 12). Lesser amounts of protein from E. coli DH5α(pZR22) produced little or no shift: the probe had been incubated with 5.2 μg of protein for the preparation run in lanes 4 and 11; 1.5 μg of protein was provided for lanes 3 and 10; 0.15 μg of protein was provided for lane 9. The tests whose results are shown in lanes 9, 10, 11, and 12 included 1.5 mM protocatechuate in the assay. Binding of the probe to 15 μg of protein contained in an extract from E. coli DH5α(pZR22) appears to have been competitively removed by 1.8 μg of pZR23 DNA (which contains the 216-bp _Xmn_I-_Eco_RI segment [lane 7]) and not by 1.8 μg of pWH661+ DNA (which is the pZR22 vector lacking the insert [lane 8]).
FIG. 8
Consensus nucleotide sequences at DNA locations where transcription is regulated by metabolites in the β-ketoadipate pathway. The nucleotide sequence of the 216-bp _Xmn_I-_Eco_RI fragment that binds PcaU contains a segment that displays identity to the pobR operator at 16 of 19 positions. The pobR operator is also closely similar to putative operators that are governed by PcaR and the inducer β-ketoadipate in P. putida. A scan for similar nucleotide sequences in the 20-kb Acinetobacter pca-qui-pob cluster revealed an additional close match directly downstream from pcaU. Arrows, inverted repetitions conserved in the consensus sequence.
FIG. 9
Binding of PcaU to DNA directly downstream from pcaU. Probe (10 ng per reaction mix) was preincubated with 10 μg of protein from E. coli DH5α(pZR22) (lanes 1 through 7) or from E. coli DH5α(pWH661+) (lane 8) or without protein (lane 9). Additional components were as follows: 50, 30, and 10 ng of an unlabelled 297-bp PCR DNA fragment from the unrelated vanA gene from Acinetobacter (lanes 1 through 3, respectively); and 50, 30 and 10 ng of the unlabelled PCR fragment used to construct the probe (lanes 4 through 6, respectively). As with PcaU bound to the upstream site, several protein bands were observed, suggesting that bound PcaU exists in oligomeric forms.
FIG. 10
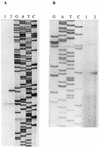
Transcriptional starts of the pca operon (A) and pcaU (B). Primer extension was used to determine the transcriptional starts. The cDNA from reverse transcription was loaded onto sequencing gels next to sequencing reactions with the same primers (lanes GATC); 10 μg of RNA was analyzed in each reaction. The RNA was isolated from cells after growth with succinate (lane 1) or _p_-hydroxybenzoate (lane 2) as the carbon source.
FIG. 11
Conserved and divergent elements in the respective genetic regions governed by PcaU and PobR. Dashed vertical lines mark sites where the pcaI-pcaU and pobA-pobR regions have been aligned. Aligned positions are the transcriptional starts of structural genes (pcaI and pobA, respectively) and the highly conserved operators. Also shown in the pcaI-pcaU region are the translational start of PcaI, the _Eco_RI site that allows separation of pcaU from the pca structural genes, the A strings that are probable locations favoring DNA bending, the location of the pcaP1 promoter, and both the transcriptional and translational starts of pobR. Comparison of these sites with the aligned pobA-pobR sequence reveals substantial differences in the functional organization of DNA. Arrows, inverted repetitions; shaded boxes, conserved residues; underlining, putative promoter sequences; circles, nucleotides replaced by pcaP1.
FIG. 12
Ancestry of regulatory proteins and the operators to which they bind. Acinetobacter PobR (9), Acinetobacter PcaU, Pseudomonas PcaR (52), and Erwinina KdgR (45) have a common ancestry and respond to chemically different effectors. E. coli GlpR (7) is a member of a different evolutionary family of regulatory proteins, but comparison of identical nucleotide residues (represented by shading) raises the possibility of a common ancestry for the glp operator and operators associated with aromatic catabolism in Acinetobacter and Pseudomonas. Arrows, inverted repetitions.
Similar articles
- Mutation analysis of PobR and PcaU, closely related transcriptional activators in acinetobacter.
Kok RG, D'Argenio DA, Ornston LN. Kok RG, et al. J Bacteriol. 1998 Oct;180(19):5058-69. doi: 10.1128/JB.180.19.5058-5069.1998. J Bacteriol. 1998. PMID: 9748437 Free PMC article. - Multiple-level regulation of genes for protocatechuate degradation in Acinetobacter baylyi includes cross-regulation.
Siehler SY, Dal S, Fischer R, Patz P, Gerischer U. Siehler SY, et al. Appl Environ Microbiol. 2007 Jan;73(1):232-42. doi: 10.1128/AEM.01608-06. Epub 2006 Nov 3. Appl Environ Microbiol. 2007. PMID: 17085716 Free PMC article. - Transcriptional organization of genes for protocatechuate and quinate degradation from Acinetobacter sp. strain ADP1.
Dal S, Trautwein G, Gerischer U. Dal S, et al. Appl Environ Microbiol. 2005 Feb;71(2):1025-34. doi: 10.1128/AEM.71.2.1025-1034.2005. Appl Environ Microbiol. 2005. PMID: 15691962 Free PMC article. - Arac/XylS family of transcriptional regulators.
Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. Gallegos MT, et al. Microbiol Mol Biol Rev. 1997 Dec;61(4):393-410. doi: 10.1128/mmbr.61.4.393-410.1997. Microbiol Mol Biol Rev. 1997. PMID: 9409145 Free PMC article. Review. - The osmotic stress response operon betIBA is under the functional regulation of BetI and the quorum-sensing regulator AnoR in Acinetobacter nosocomialis.
Subhadra B, Surendran S, Lim BR, Yim JS, Kim DH, Woo K, Kim HJ, Oh MH, Choi CH. Subhadra B, et al. J Microbiol. 2020 Jun;58(6):519-529. doi: 10.1007/s12275-020-0186-1. Epub 2020 May 27. J Microbiol. 2020. PMID: 32462489 Review.
Cited by
- Characterization of the isophthalate degradation genes of Comamonas sp. strain E6.
Fukuhara Y, Inakazu K, Kodama N, Kamimura N, Kasai D, Katayama Y, Fukuda M, Masai E. Fukuhara Y, et al. Appl Environ Microbiol. 2010 Jan;76(2):519-27. doi: 10.1128/AEM.01270-09. Epub 2009 Nov 20. Appl Environ Microbiol. 2010. PMID: 19933340 Free PMC article. - Role of Acinetobacter baylyi Crc in catabolite repression of enzymes for aromatic compound catabolism.
Zimmermann T, Sorg T, Siehler SY, Gerischer U. Zimmermann T, et al. J Bacteriol. 2009 Apr;191(8):2834-42. doi: 10.1128/JB.00817-08. Epub 2009 Feb 6. J Bacteriol. 2009. PMID: 19201803 Free PMC article. - Simultaneous catabolism of plant-derived aromatic compounds results in enhanced growth for members of the Roseobacter lineage.
Gulvik CA, Buchan A. Gulvik CA, et al. Appl Environ Microbiol. 2013 Jun;79(12):3716-23. doi: 10.1128/AEM.00405-13. Epub 2013 Apr 5. Appl Environ Microbiol. 2013. PMID: 23563956 Free PMC article. - Characterization of the genes for two protocatechuate 3, 4-dioxygenases from the 4-sulfocatechol-degrading bacterium Agrobacterium radiobacter strain S2.
Contzen M, Stolz A. Contzen M, et al. J Bacteriol. 2000 Nov;182(21):6123-9. doi: 10.1128/JB.182.21.6123-6129.2000. J Bacteriol. 2000. PMID: 11029433 Free PMC article. - NpdR, a repressor involved in 2,4,6-trinitrophenol degradation in Rhodococcus opacus HL PM-1.
Nga DP, Altenbuchner J, Heiss GS. Nga DP, et al. J Bacteriol. 2004 Jan;186(1):98-103. doi: 10.1128/JB.186.1.98-103.2004. J Bacteriol. 2004. PMID: 14679229 Free PMC article.
References
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1994.
- Canovas J L, Stanier R Y. Regulation of the enzymes of the β-ketoadipate pathway in Moraxella calcoacetica. I. General aspects. Eur J Biochem. 1967;1:289–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources