Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells - PubMed (original) (raw)
Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells
Z Yan et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Cdc6 has a critical regulatory role in the initiation of DNA replication in yeasts, but its function in mammalian cells has not been characterized. We show here that Cdc6 is expressed selectively in proliferating but not quiescent mammalian cells, both in culture and within tissues of intact animals. During the transition from a growth-arrested to a proliferative state, transcription of mammalian Cdc6 is regulated by E2F proteins, as revealed by a functional analysis of the human Cdc6 promoter and by the ability of exogenously expressed E2F proteins to stimulate the endogenous Cdc6 gene. Immunodepletion of Cdc6 by microinjection of anti-Cdc6 antibody blocks initiation of DNA replication in a human tumor cell line. We conclude that expression of human Cdc6 is regulated in response to mitogenic signals though transcriptional control mechanisms involving E2F proteins, and that Cdc6 is required for initiation of DNA replication in mammalian cells.
Figures
Figure 1
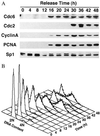
Induction of Cdc6 in human cells during release from contact inhibition. (A) Immunoblot analysis of Cdc6 expression in relation to other proteins at specific time points during entry into the cell cycle. Human T24 cells were plated at high density (4 × 106 cells/150 mm) in McCoy 5A media supplemented with 10% fetal bovine serum and grown for 4 days. To release cells from contact inhibition, they were trypsinized and replated at lower density (1 × 106 cells/150 mm) in the same media. (B) Flow cytometric analysis of DNA content in T24 cells samples at specific time points after release from contact inhibition. The results show that contact-inhibited cells are arrested with unreplicated DNA (2N), and that cells enter S phase between 20 and 24 hr after release.
Figure 2
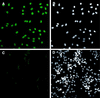
Immunofluorescence staining of Cdc6 in proliferating human cells. Cdc6 is localized to the nucleus of asynchronously proliferating T24 cells (A) but is not detected in contact-inhibited cells (C). Propidium iodide staining (Right) identifies all the nuclei present within the same fields (B and D).
Figure 3
Selective expression of Cdc6 in proliferating cell populations within intact mammalian tissues. In situ hybridization of sections of duodenum from an adult mouse by using antisense (A and B) or sense (C and D) probes, transcribed in vitro from Cdc6 cDNA. The boxed areas in A and C are shown at higher magnification in B and D. (Bar = 100 μm.)
Figure 4
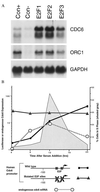
(A) Quiescent, serum-deprived human foreskin fibroblasts (passage 7) were infected with the indicated recombinant adenoviruses, each at an multiplicity of infection of 300, and then harvested for Northern blot analysis at 15 hr postinfection. Blots were hybridized with a human Cdc6 probe, or with a glyceraldehyde-3-phosphate dehydrogenase probe as a control for RNA loading. The samples labeled as E2F1, E2F2, or E2F3 represent infections with the indicated E2F viruses together with an adenovirus expressing DP1 to provide the dimeric partner for E2F. (B) E2F binding sites are required for correct regulation of the human Cdc6 promoter. Transient transfection assays were performed in subconfluent 3T3 cells under conditions of growth arrest induced by serum deprivation (t = 0 hr), and at subsequent time points after readdition of serum. Reporter gene activity (mean of triplicate determinations) was calculated relative to expression of a cytomegalovirus-luciferase gene in sister cultures assayed at each time interval, and displayed in relation to endogenous Cdc6 mRNA (Northern blot analysis; calculated as multiples of the hybridization signal at t = 0 hr) and to the fraction of cells in S phase (flow cytometry). Similar results were obtained in each of four separate transfection assays. A schematic representation of the human Cdc6 promoter illustrates E2F binding sites in relation to the transcriptional start site (arrow) of the human Cdc6 gene (14), and disruption of these sites (X) in the mutated promoter.
Figure 5
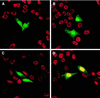
Immunodepletion of Cdc6 blocks DNA replication in HeLa cells. Metaphase cells were microinjected with affinity purified anti-Cdc6 (A–C) or control non-immune IgG (D), each at a concentration of 1.5 μg/μl, and cultured subsequently for 18 hr in the presence of bromodeoxyuridine (BrdUrd). Microinjected antibodies (green) and nuclear incorporation of BrdUrd (red) were detected by immunofluoresence microscopy. The nuclei of microinjected cells that incorporate BrdUrd appear yellow in the double immunofluorescence images.
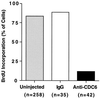
Figure 6
Quantitative analysis of BrdUrd incorporation in uninjected cells and in cells microinjected with the indicated antibodies. The number of cells scored for DNA replication in each test condition is indicated.
Similar articles
- Regulation of cell growth-dependent expression of mammalian CDC6 gene by the cell cycle transcription factor E2F.
Ohtani K, Tsujimoto A, Ikeda M, Nakamura M. Ohtani K, et al. Oncogene. 1998 Oct 8;17(14):1777-85. doi: 10.1038/sj.onc.1202105. Oncogene. 1998. PMID: 9778043 - Cell cycle-regulated expression of mammalian CDC6 is dependent on E2F.
Hateboer G, Wobst A, Petersen BO, Le Cam L, Vigo E, Sardet C, Helin K. Hateboer G, et al. Mol Cell Biol. 1998 Nov;18(11):6679-97. doi: 10.1128/MCB.18.11.6679. Mol Cell Biol. 1998. PMID: 9774682 Free PMC article. - Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F.
Ohtani K, Iwanaga R, Nakamura M, Ikeda M, Yabuta N, Tsuruga H, Nojima H. Ohtani K, et al. Oncogene. 1999 Apr 8;18(14):2299-309. doi: 10.1038/sj.onc.1202544. Oncogene. 1999. PMID: 10327050 - Cell-cycle regulation of gene expression by transcriptional repression.
Zwicker J, Müller R. Zwicker J, et al. Trends Genet. 1997 Jan;13(1):3-6. doi: 10.1016/s0168-9525(96)30112-1. Trends Genet. 1997. PMID: 9009839 Review. No abstract available. - E2F target genes and cell-cycle checkpoint control.
Lavia P, Jansen-Dürr P. Lavia P, et al. Bioessays. 1999 Mar;21(3):221-30. doi: 10.1002/(SICI)1521-1878(199903)21:3<221::AID-BIES6>3.0.CO;2-J. Bioessays. 1999. PMID: 10333731 Review.
Cited by
- Human DNA helicase B interacts with the replication initiation protein Cdc45 and facilitates Cdc45 binding onto chromatin.
Gerhardt J, Guler GD, Fanning E. Gerhardt J, et al. Exp Cell Res. 2015 Jun 10;334(2):283-93. doi: 10.1016/j.yexcr.2015.04.014. Epub 2015 Apr 29. Exp Cell Res. 2015. PMID: 25933514 Free PMC article. - Estrogen-induced upregulation and 3'-UTR shortening of CDC6.
Akman BH, Can T, Erson-Bensan AE. Akman BH, et al. Nucleic Acids Res. 2012 Nov;40(21):10679-88. doi: 10.1093/nar/gks855. Epub 2012 Sep 12. Nucleic Acids Res. 2012. PMID: 22977174 Free PMC article. - HBx protein of hepatitis B virus promotes reinitiation of DNA replication by regulating expression and intracellular stability of replication licensing factor CDC6.
Pandey V, Kumar V. Pandey V, et al. J Biol Chem. 2012 Jun 8;287(24):20545-54. doi: 10.1074/jbc.M112.359760. Epub 2012 Apr 19. J Biol Chem. 2012. PMID: 22523071 Free PMC article. - The Cdc6 nucleotide-binding site regulates its activity in DNA replication in human cells.
Herbig U, Marlar CA, Fanning E. Herbig U, et al. Mol Biol Cell. 1999 Aug;10(8):2631-45. doi: 10.1091/mbc.10.8.2631. Mol Biol Cell. 1999. PMID: 10436018 Free PMC article. - Analysis of Cdc6 function in the assembly of mammalian prereplication complexes.
Cook JG, Park CH, Burke TW, Leone G, DeGregori J, Engel A, Nevins JR. Cook JG, et al. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1347-52. doi: 10.1073/pnas.032677499. Epub 2002 Jan 22. Proc Natl Acad Sci U S A. 2002. PMID: 11805305 Free PMC article.
References
- Stillman B. Science. 1996;274:1659–1664. - PubMed
- Bell S P, Stillman B. Nature (London) 1992;357:128–134. - PubMed
- Kelly T J, Martin G S, Forsburg S L, Stephen R J, Russo A, Nurse P. Cell. 1993;74:371–382. - PubMed
- Liang C, Weinreich M, Stillman B. Cell. 1995;81:667–676. - PubMed
- Jallepalli P, Kelly T. Genes Dev. 1996;10:541–552. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials