The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death - PubMed (original) (raw)
The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death
C E Bazenet et al. Proc Natl Acad Sci U S A. 1998.
Abstract
An increase in the level of the c-Jun transcription factor and of its phosphorylation has previously been shown to be essential for nerve growth factor (NGF) withdrawal-induced apoptosis of rat sympathetic neurons (SCG). The Rho-like GTPases Cdc42 and Rac1 are involved in the regulation of a number of cellular processes, including activation of the c-Jun NH2-terminal kinase (JNK) pathway. Therefore, we have investigated the role of these GTPases in this process. Overexpression of activated Rac1 or Cdc42 in SCG neurons maintained in the presence of NGF induced apoptosis, whereas expression of dominant negative mutants of Cdc42 or Rac1 blocked apoptosis following NGF withdrawal. Cdc42 activation produced an increase in the level of c-Jun and of its phosphorylation. Furthermore, Cdc42-induced death was prevented by coexpressing the c-Jun dominant negative FLAGDelta169. Thus, Cdc42 appears to function as an initiator of neuronal cell death by activating a transcriptional pathway regulated by c-Jun.
Figures
Figure 1
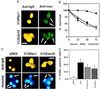
Activated Cdc42 and Rac1 induce neuronal apoptosis. (A) The activated V12 mutants of Cdc42 and Rac1 were subcloned into the pRK5 mammalian expression vector and tagged with a myc epitope. Sympathetic neurons (SCG neurons), cultured for 5–7 days in the presence of NGF, were microinjected with 0.1 mg/ml DNA and 5 mg/ml guinea pig IgG, to follow the injected cells. For each experiment, 200 cells were microinjected. Four to 24 hr after injection, the cells were stained to check for expression of Rac1 and Cdc42 as described in Materials and Methods. (Bar = 100 μm.) The white arrows indicate the expressing cells. (B) Survival of SCG neurons injected with V12Cdc42 (triangles), V12Rac1 (squares), or pRK5 (circles). Twenty-four, 48, and 72 hr later, the percentage of surviving cells was assessed as described in Material and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments ± SEM. (C) Morphology of the cells microinjected with V12Cdc42, V12Rac1, or pRK5. Six-day-old SCG neurons were injected with 0.3 mg/ml DNA together with guinea pig IgG and stained with Hoechst 24 hr after injection. White arrows indicate the injected cells: only the cells overexpressing the constitutively active forms of Rac1 and Cdc42 displayed clearly pyknotic nuclei. (Bar = 100 μm.) (D) TUNEL analysis of SCG neurons microinjected with V12Cdc42, V12Rac1, pRK5, or Bax. Six-day-old SCG neurons were microinjected with 0.3 mg/ml V12 Rac1, V12Cdc42, pRK5 DNA, or 0.05 mg/ml Bax DNA. TUNEL analysis was performed 16 hr later. The results are the means of three independent experiments ± SEM.
Figure 2
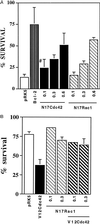
N17Rac1 and N17Cdc42 can prevent NGF withdrawal-induced neuronal death. (A) SCG neurons, cultured for 5–7 days, were microinjected with increasing concentrations of N17Rac1 (hatched bars), N17Cdc42 (solid bars), 1.0 mg/ml pRK5 (negative control, open bar), or 0.05 mg/ml of Bcl-2 (positive control, striped bar). Twenty-four hours later the cells were withdrawn from NGF and left for an additional 48 hr. Cell survival was assessed by calcein staining as described in Material and Methods. The results are the means of four independent experiments ± SEM. #, the P value of N17Cdc42 at 0.1 mg/ml is <0.01. (B) Sympathetic neurons (SCG neurons), cultured for 5–7 days in the presence of NGF, were coinjected with 0.1 mg/ml V12Cdc42 (solid bar) and increasing concentrations of N17Rac1 (hatched bars) or 0.4 mg/ml pRK5 (open bar). Forty-eight hours later, the percentage of surviving cells was assessed as described in Material and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments ± SEM.
Figure 3
Activation of Cdc42 results in an increase in the level of c-Jun protein and of its phosphorylation. (A) V12Cdc42 or pRK5 (0.3 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, which were maintained in the presence of NGF. Twenty-four hours after injection, the cells were fixed, permeabilized, and stained with Hoechst dye (Left), a rhodamine-conjugated anti-guinea pig IgG antibody to detect the injected cells (Center), and an anti-c-Jun antibody (Right). Only the cells in which c-Jun staining was clearly above background were scored as positive. The results are presented as a scatter plot (Upper). The data are the means ± SEM of six independent experiments. Cdc42 induced a 2-fold increase in the percent of cells expressing c-Jun (white arrows) (P < 0.02). (Bar = 100 μm.) (B) V12Cdc42 or pRK5 (0.3 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, which were kept in the presence of NGF. Twenty-four hours after injection, the percent of cells expressing phospho-c-Jun was assessed. Only the cells in which phospho-c-Jun staining was clearly above background were scored positive. The results are presented as a bar graph (Upper). The data are the means ± SEM of four independent experiments; P < 0.03. Cdc42 is capable of inducing a significant increase in the level of phospho-c-Jun in the injected cells (white arrows). (Bar = 100 μm.) (C) N17Cdc42 or pRK5 (0.6 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, together with guinea pig IgG. The neurons were withdrawn from NGF 4–6 hr after injection. Twenty-four hours later, the percentage of cells expressing nuclear c-Jun was assessed. The results were represented as a bar graph. The data are the means ± SEM of six independent experiments. N17Cdc42 can block the induction of the increase in the level of c-Jun that is normally observed after NGF withdrawal (white arrows). (Bar = 100 μm.)
Figure 4
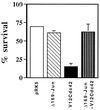
Cdc42-induced apoptosis requires AP-1 activity. pCDFLAGΔ169 (0.4 mg/ml), 0.1 mg/ml V12Cdc42, and 70 kDa Texas Red Dextran were coinjected into SCG neurons. The cells were maintained in the presence of NGF, and the percentage of surviving cells was assessed 48 hr later. The results are the means of three independent experiments ± SEM. FLAGΔ169 blocked V12Cdc42-induced death.
Similar articles
- Evidence for a role of mixed lineage kinases in neuronal apoptosis.
Mota M, Reeder M, Chernoff J, Bazenet CE. Mota M, et al. J Neurosci. 2001 Jul 15;21(14):4949-57. doi: 10.1523/JNEUROSCI.21-14-04949.2001. J Neurosci. 2001. PMID: 11438570 Free PMC article. - Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons.
Kanamoto T, Mota M, Takeda K, Rubin LL, Miyazono K, Ichijo H, Bazenet CE. Kanamoto T, et al. Mol Cell Biol. 2000 Jan;20(1):196-204. doi: 10.1128/MCB.20.1.196-204.2000. Mol Cell Biol. 2000. PMID: 10594022 Free PMC article. - The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis.
Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. Xu Z, et al. Mol Cell Biol. 2001 Jul;21(14):4713-24. doi: 10.1128/MCB.21.14.4713-4724.2001. Mol Cell Biol. 2001. PMID: 11416147 Free PMC article. - The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes.
Auer KL, Contessa J, Brenz-Verca S, Pirola L, Rusconi S, Cooper G, Abo A, Wymann MP, Davis RJ, Birrer M, Dent P. Auer KL, et al. Mol Biol Cell. 1998 Mar;9(3):561-73. doi: 10.1091/mbc.9.3.561. Mol Biol Cell. 1998. PMID: 9487126 Free PMC article. - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
Cited by
- Evidence for a role of mixed lineage kinases in neuronal apoptosis.
Mota M, Reeder M, Chernoff J, Bazenet CE. Mota M, et al. J Neurosci. 2001 Jul 15;21(14):4949-57. doi: 10.1523/JNEUROSCI.21-14-04949.2001. J Neurosci. 2001. PMID: 11438570 Free PMC article. - Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production.
Embade N, Valerón PF, Aznar S, López-Collazo E, Lacal JC. Embade N, et al. Mol Biol Cell. 2000 Dec;11(12):4347-58. doi: 10.1091/mbc.11.12.4347. Mol Biol Cell. 2000. PMID: 11102528 Free PMC article. - Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration.
Li R, Li D, Wu C, Ye L, Wu Y, Yuan Y, Yang S, Xie L, Mao Y, Jiang T, Li Y, Wang J, Zhang H, Li X, Xiao J. Li R, et al. Theranostics. 2020 Jan 1;10(4):1649-1677. doi: 10.7150/thno.40919. eCollection 2020. Theranostics. 2020. PMID: 32042328 Free PMC article. - Inhibition of Rac GTPase triggers a c-Jun- and Bim-dependent mitochondrial apoptotic cascade in cerebellar granule neurons.
Le SS, Loucks FA, Udo H, Richardson-Burns S, Phelps RA, Bouchard RJ, Barth H, Aktories K, Tyler KL, Kandel ER, Heidenreich KA, Linseman DA. Le SS, et al. J Neurochem. 2005 Aug;94(4):1025-39. doi: 10.1111/j.1471-4159.2005.03252.x. J Neurochem. 2005. PMID: 16092944 Free PMC article.
References
- Oppenheim R W. Annu Rev Neurosci. 1991;14:453–501. - PubMed
- Linnik M D. Rest Neurol Neurosci. 1996;9:219–225. - PubMed
- Barde Y. Neuron. 1989;2:1525–1534. - PubMed
- Snider W D. Cell. 1994;77:627–638. - PubMed
- Kaplan D R, Miller F D. Curr Biol. 1997;9:213–221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous