Profound neuronal plasticity in response to inactivation of the dopamine transporter - PubMed (original) (raw)
Profound neuronal plasticity in response to inactivation of the dopamine transporter
S R Jones et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The dopamine transporter (DAT) plays an important role in calibrating the duration and intensity of dopamine neurotransmission in the central nervous system. We have used a strain of mice in which the gene for the DAT has been genetically deleted to identify the DAT's homeostatic role. We find that removal of the DAT dramatically prolongs the lifetime (300 times) of extracellular dopamine. Within the time frame of neurotransmission, no other processes besides diffusion can compensate for the lack of the DAT, and the absence of the DAT produces extensive adaptive changes to control dopamine neurotransmission. Despite the absence of a clearance mechanism, dopamine extracellular levels were only 5 times greater than control animals due to a 95% reduction in content and a 75% reduction in release. Paradoxically, dopamine synthesis rates are doubled despite a decrease of 90% in the levels of tyrosine hydroxylase and degradation is markedly enhanced. Thus, the DAT not only controls the duration of extracellular dopamine signals but also plays a critical role in regulating presynaptic dopamine homeostasis. It is interesting to consider that the switch to a dopamine-deficient, but functionally hyperactive, mode of neurotransmission observed in mice lacking the DAT may represent an extreme example of neuronal plasticity resulting from long-term psychostimulant abuse.
Figures
Figure 1
Time course of dopamine release and uptake in mouse striatal slices. (Upper) Individual recordings of dopamine efflux evoked by a single electrical pulse in striatal slices from control (DAT+/+), heterozygote (DAT+/−), and homozygote (DAT−/−) mice as measured by cyclic voltammetry (12) (○, data; solid lines, simulations). Pseudo-first order rate constants (k) for uptake are shown in inset. (Lower) Effects of various drugs on stimulated dopamine efflux in striatal slices from DAT−/− animals. Results after drug application are superimposed on the predrug response. All drugs were applied at a concentration of 10 μM for 20 min, and none caused any measurable effect. Results are representative of recordings from at least 4 different animals.
Figure 2
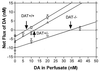
Basal extracellular concentrations of dopamine measured in vivo. Concentrations of dopamine in the striatum of freely moving mice determined using a quantitative microdialysis method (16, 17). Data are given as the mean ± SEM of the difference between the concentration of dopamine applied to the dialysis probe in the perfusate and that collected at the probe effluent. The point of zero flux (“no net flux”), extrapolated by linear regression, represents the concentration of dopamine at which diffusion into the probe equals that out of the probe. These values, that provide the basal extracellular concentration of dopamine, were 6.95 ± 0.51 nM for DAT+/+; 12.2 ± 0.79 nM for DAT+/−, and 34.5 ± 3.22 nM for DAT−/− mice. Four animals were measured in each group. The slope of the lines yield the apparent recovery of dopamine from the brain. Recovery is much lower in the DAT−/− (29 ± 5%) than in wild-type (54 ± 5%) or heterozygote (52 ± 4%) animals, in accord with theory for systems without active transport (16).
Figure 3
Tissue content of monoamines and metabolites. Amounts of monoamines and metabolites were measured in striatal homogenates. DOPAC/dopamine ratios were 0.06, 0.08, and 1.6 for DAT+/+, DAT+/−, and DAT−/− mice, respectively, and HVA/dopamine ratios were 0.09, 0.3, and 4.3, respectively. Results are presented as mean ± SEM of determinations from 10–12 animals. An asterisk (∗) indicates value is significantly different (P < 0.05) from corresponding value in DAT+/+ mice as determined by Student’s t test.
Figure 4
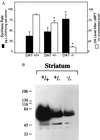
(A) Indices of dopamine synthesis. Synthesis rates of dopamine (solid bars) and effect of a tyrosine hydroxylase inhibitor (2) (αMPT) on tissue levels of dopamine (open bars) in normal (DAT+/+), heterozygote (DAT+/−), and homozygote mice (DAT−/−). Results are presented as the mean ± SEM of determinations from 4 to 6 different animals per genotype. (B) Western blot of TH in the striatum of wild-type and mutant mice. TH labeling decreased by 59% and 96% in heterozygote and homozygote striata, respectively, as compared with wild-type values. Immunohistochemistry with a TH antibody demonstrates that the decrease in TH protein levels is not due to the destruction of dopamine neurons as these were present at normal levels (M.J. et al., unpublished observation). An asterisk (∗) indicates value is significantly different (P < 0.05) from corresponding value in DAT+/+ mice as determined by Student’s t test.
Similar articles
- Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis.
Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Gainetdinov RR, et al. Brain Res Brain Res Rev. 1998 May;26(2-3):148-53. doi: 10.1016/s0165-0173(97)00063-5. Brain Res Brain Res Rev. 1998. PMID: 9651511 Review. - Phenotypic expression of the targeted null-mutation in the dopamine transporter gene varies as a function of the genetic background.
Morice E, Denis C, Giros B, Nosten-Bertrand M. Morice E, et al. Eur J Neurosci. 2004 Jul;20(1):120-6. doi: 10.1111/j.1460-9568.2004.03465.x. Eur J Neurosci. 2004. PMID: 15245485 - Presynaptic control of striatal dopamine neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice.
Patel J, Mooslehner KA, Chan PM, Emson PC, Stamford JA. Patel J, et al. J Neurochem. 2003 May;85(4):898-910. doi: 10.1046/j.1471-4159.2003.01732.x. J Neurochem. 2003. PMID: 12716422 - Increased rewarding properties of morphine in dopamine-transporter knockout mice.
Spielewoy C, Gonon F, Roubert C, Fauchey V, Jaber M, Caron MG, Roques BP, Hamon M, Betancur C, Maldonado R, Giros B. Spielewoy C, et al. Eur J Neurosci. 2000 May;12(5):1827-37. doi: 10.1046/j.1460-9568.2000.00063.x. Eur J Neurosci. 2000. PMID: 10792459 Free PMC article. - [Behavioral, cellular and molecular consequences of the dopamine transporter gene inactivation].
Jaber M, Bloch B, Caron MG, Giros B. Jaber M, et al. C R Seances Soc Biol Fil. 1998;192(6):1127-37. C R Seances Soc Biol Fil. 1998. PMID: 10101608 Review. French.
Cited by
- Enhanced tyrosine hydroxylase activity induces oxidative stress, causes accumulation of autotoxic catecholamine metabolites, and augments amphetamine effects in vivo.
Vecchio LM, Sullivan P, Dunn AR, Bermejo MK, Fu R, Masoud ST, Gregersen E, Urs NM, Nazari R, Jensen PH, Ramsey A, Goldstein DS, Miller GW, Salahpour A. Vecchio LM, et al. J Neurochem. 2021 Aug;158(4):960-979. doi: 10.1111/jnc.15432. Epub 2021 Jun 12. J Neurochem. 2021. PMID: 33991113 Free PMC article. - Identification of a Vav2-dependent mechanism for GDNF/Ret control of mesolimbic DAT trafficking.
Zhu S, Zhao C, Wu Y, Yang Q, Shao A, Wang T, Wu J, Yin Y, Li Y, Hou J, Zhang X, Zhou G, Gu X, Wang X, Bustelo XR, Zhou J. Zhu S, et al. Nat Neurosci. 2015 Aug;18(8):1084-93. doi: 10.1038/nn.4060. Epub 2015 Jul 6. Nat Neurosci. 2015. PMID: 26147533 - Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter.
Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Chen R, et al. Proc Natl Acad Sci U S A. 2006 Jun 13;103(24):9333-8. doi: 10.1073/pnas.0600905103. Epub 2006 Jun 5. Proc Natl Acad Sci U S A. 2006. PMID: 16754872 Free PMC article. - Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity.
Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Morón JA, et al. J Neurosci. 2003 Sep 17;23(24):8480-8. doi: 10.1523/JNEUROSCI.23-24-08480.2003. J Neurosci. 2003. PMID: 13679416 Free PMC article. - Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia.
Morice E, Billard JM, Denis C, Mathieu F, Betancur C, Epelbaum J, Giros B, Nosten-Bertrand M. Morice E, et al. Neuropsychopharmacology. 2007 Oct;32(10):2108-16. doi: 10.1038/sj.npp.1301354. Epub 2007 Mar 7. Neuropsychopharmacology. 2007. PMID: 17342172 Free PMC article.
References
- Amara S G, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. - PubMed
- Feldman R S, Meyer J S, Quenzer L F. Principles of Neuropsycho-Pharmacology. Sunderland, MA: Sinauer; 1997. , Chapt. 8 and 9.
- Horn A S. Prog Neurobiol. 1990;34:387–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DA005749/DA/NIDA NIH HHS/United States
- R01 NS019576/NS/NINDS NIH HHS/United States
- F32 DA05749-01/DA/NIDA NIH HHS/United States
- T32 AG000029/AG/NIA NIH HHS/United States
- DA 10900/DA/NIDA NIH HHS/United States
- R01 DA010900/DA/NIDA NIH HHS/United States
- 5 T32 AG00029-20/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous