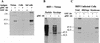Bovine herpesvirus 1 glycoprotein M forms a disulfide-linked heterodimer with the U(L)49.5 protein - PubMed (original) (raw)
Bovine herpesvirus 1 glycoprotein M forms a disulfide-linked heterodimer with the U(L)49.5 protein
S X Wu et al. J Virol. 1998 Apr.
Abstract
Nine glycoproteins (gB, gC, gD, gE, gG, gH, gI, gK, and gL) have been identified in bovine herpesvirus 1 (BHV-1). gM has been identified in many other alpha-, beta-, and gammaherpesviruses, in which it appears to play a role in membrane penetration and cell-to-cell fusion. We sought to express BHV-1 open reading frame U(L)10, which encodes gM, and specifically identify the glycoprotein. We corrected a frameshift error in the published sequence and used the corrected sequence to design coterminal peptides from the C terminus. These were expressed as glutathione S-transferase fusion proteins in Escherichia coli. The fusion protein containing the 63 C-terminal amino acids from the corrected gM sequence engendered antibodies that immunoprecipitated a 30-kDa protein from in vitro translation reactions programmed with the U(L)10 gene. Proteins immunoprecipitated by this antibody from virus-infected cells ran at 36 and 43 kDa in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and 43 and 48 kDa in nonreducing SDS-PAGE. Only the larger of the pair was present in virions. A 7-kDa protein was released from gM by reducing agents. The 7-kDa protein was not recognized in Western blots probed with the anti-gM antibody but reacted specifically with antibodies prepared against BHV-1 U(L)49.5, previously reported to be a 9-kDa protein associated with an unidentified 39-kDa protein (X. Liang, B. Chow, C. Raggo, and L. A. Babiuk, J. Virol. 70:1448-1454, 1996). This is the first report of a small protein covalently bound to any herpesvirus gM. Similar patterns of hydrophobic domains and cysteines in all known gM and U(L)49.5 homologs suggest that these two proteins may be linked by disulfide bonds in all herpesviruses.
Figures
FIG. 1
Antibodies (Ab) against the 3′ end of BHV-1 UL10 immunoprecipitate the UL10 in vitro translation product. (A) A 30-kDa protein was synthesized in a reticulocyte lysate in the presence but not the absence of the UL10 RNA transcript. The sample was treated at 56°C for 10 min in the presence of reducing agent, subjected SDS-PAGE in a 12% gel, and autoradiographed. (B) Sera from mice immunized with gMC-63 precipitated the 30-kDa protein synthesized in an in vitro translation reaction programmed with UL10 mRNA. Sera from mice immunized with GST did not precipitate the 30-kDa protein.
FIG. 2
BHV-1 gM can be immunoprecipitated from virions, infected cells, virion envelopes, and the membranes but not the cytosol of infected cells. (A) MDBK cells were infected (Inf) at an MOI of 10 or mock infected and labeled with [35S]methionine and -cysteine for 16 h beginning 6 h after infection. Labeled virions were semipurified by centrifugation through a 30% sucrose cushion. The cells and virions were lysed with NP-40 and sodium deoxycholate. Samples were immunoprecipitated with the gMC or GST antibody (Ab), treated at 56°C with SDS-PAGE sample buffer in the presence of the reducing agent DTT, analyzed by SDS-PAGE on a 12% gel, and autoradiographed. (B) Metabolically radiolabeled virions were lysed in NP-40 and sodium deoxycholate and either loaded directly on the gel (−) or immunoprecipitated with gMC antibody (+). Another sample of virus was treated with detergents, and the envelope fraction was cleared of nucleocapsids by centrifugation over a 30% sucrose cushion and either loaded directly on the gel (−) or precipitated with gMC antibody (+). Samples were treated with DTT at 56°C, analyzed by SDS-PAGE on a 12% gel, and autoradiographed. (C) Cells were infected and labeled as for panel A. Cell membranes were obtained from cells disrupted in a Dounce homogenizer, centrifuged at low speed to remove cell debris, pelleted at 12,000 rpm, washed with homogenizing buffer, and repelleted at 40,000 rpm. Lysates of total cells, the 40K supernatant from washed membranes (40KSupe), and the washed membranes themselves were immunoprecipitated with antibody against GST or gM. Precipitates were treated with DTT at 56°C, analyzed by SDS-PAGE on a 12% gel, and autoradiographed.
FIG. 3
Deglycosylation of gM by PNGase F and endo H. Lysates of 35S-labeled infected cell membranes were immunoprecipitated with gMC antibody. Precipitates were treated with 0.8% SDS at 100 or 56°C and digested with 0 to 5 kU of PNGase F or 0 to 2 mU of endo H. The gM in the left lane of each panel was synthesized in an in vitro translation system programmed with the BHV-1 UL10 RNA transcript.
FIG. 4
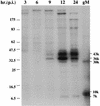
Coprecipitation of gM and a 7-kDa protein by gMC antibody at various times postinfection (p.i.). MDBK cells were infected at an MOI of 10 and labeled with [35S]methionine and -cysteine for 1 h at the times indicated. Cells were lysed and immunoprecipitated with gMC antibody. The samples were analyzed by SDS-PAGE in a 10 to 20% gradient gel alongside in vitro-translated BHV-1 gM (gM). The 43-, 36-, 30-, 10-, and 7-kDa protein bands are indicated.
FIG. 5
Pulse-chase and immunoprecipitation to show the maturation of BHV-1 gM. Proteins from 35S-labeled BHV-1-infected cells without (lanes 1 to 4 and 8 to 11) or with (lanes 5 and 12) tunicamycin treatment (Tunic), and similarly labeled uninfected cells (lanes 6 and 13), were chased into mature proteins for 0 to 60 min with unlabeled amino acids. gM was immunoprecipitated with gMC antibody and analyzed by SDS-PAGE on 10 to 20% gradient gels in the presence (lanes 1 to 7) or absence (lanes 8 to 14) of the reducing agent DTT. Unprecipitated UL10 in vitro translation products were analyzed in lanes 7 and 14. The strong signal at 8 to 10 kDa in the UL10 in vitro translation reaction was not present in reactions done in other reticulocyte lysates.
FIG. 6
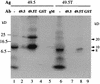
Immunoprecipitation of proteins encoded by full-length (49.5) and N-terminally truncated UL49.5 (49.5T) synthesized in vitro with antibodies (Ab) directed against fusion proteins containing the full-length UL49.5 protein (lanes 2 and 7), the truncated protein (lanes 3 and 8), GST (lanes 4 and 9), or gM (lane 5). For comparison, 1 μl of the unprecipitated UL49.5 translation reaction (lane 1) and 5 μl of the UL49.5T translation reaction (lane 6) were loaded. Each immunoprecipitation was begun with 5 μl of translation reaction mixture. Samples were analyzed by SDS-PAGE in an 18% gel and autoradiographed. Ag, antigen.
FIG. 7
Immunoprecipitation of the gM-UL49.5 complex with antibody against either gM or UL49.5. Lysates of radiolabeled uninfected cells (Un), BHV-1-infected cells (Cl), cell membranes (Cm), 40K cell membrane supernatants (Cs), and semipurified virions (V) were precipitated with antibody (Ab) against truncated UL49.5T or gM. Uninfected cells (lanes 1 and 10) were immunoprecipitated with antibodies against both gM and UL49.5. The immunoprecipitates were incubated at 56°C in the presence (lanes 1 to 9) or absence (lanes 10 to 18) of DTT, analyzed by SDS-PAGE in 10 to 20% gradient gels, and autoradiographed. Positions of molecular mass markers (left) and calculated molecular masses of antibody-precipitated bands (right), both in kilodaltons, are indicated. Ag, antigen.
FIG. 8
Analysis of the gM-UL49.5 complex by Western blotting. Cells were infected at an MOI of 10 and collected 24 h later, and membranes were prepared (Cm). Mock-infected cells were collected at the same time, and membranes were similarly prepared (U). Virions were prepared from cells infected for 60 h at an MOI of 0.5 and either semipurified (Vs) or banded on a potassium tartrate gradient (Vt). Lysates were incubated at 56°C in the presence (lanes 1 to 8) or absence (lanes 9 to 16) of 40 mM DTT for 10 min, analyzed by SDS-PAGE in 10 to 20% gradient gels, and transferred to nitrocellulose paper. The blots were probed with UL49.5T antibody (Ab) first. Bound antibody was detected by ECL (Amersham). The blots were stripped, reprobed with gM antibody, and again detected by ECL. Positions of molecular mass markers on the (left) and calculated sizes of specific bands are indicated on the (right), both in kilodaltons, are indicated. Ag, antigen.
Similar articles
- Bovine herpesvirus 1 UL49.5 homolog gene encodes a novel viral envelope protein that forms a disulfide-linked complex with a second virion structural protein.
Liang X, Chow B, Raggo C, Babiuk LA. Liang X, et al. J Virol. 1996 Mar;70(3):1448-54. doi: 10.1128/JVI.70.3.1448-1454.1996. J Virol. 1996. PMID: 8627662 Free PMC article. - Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH.
Khattar SK, van Drunen Littel-van den Harke S, Attah-Poku SK, Babiuk LA, Tikoo SK. Khattar SK, et al. Virology. 1996 May 1;219(1):66-76. doi: 10.1006/viro.1996.0223. Virology. 1996. PMID: 8623555 - Bovine herpesvirus 1 U(s) open reading frame 4 encodes a glycoproteoglycan.
Keil GM, Engelhardt T, Karger A, Enz M. Keil GM, et al. J Virol. 1996 May;70(5):3032-8. doi: 10.1128/JVI.70.5.3032-3038.1996. J Virol. 1996. PMID: 8627780 Free PMC article. - Molecular virology of ruminant herpesviruses.
Schwyzer M, Ackermann M. Schwyzer M, et al. Vet Microbiol. 1996 Nov;53(1-2):17-29. doi: 10.1016/s0378-1135(96)01231-x. Vet Microbiol. 1996. PMID: 9010995 Review. - The role of herpes simplex virus glycoproteins in the virus replication cycle.
Rajcáni J, Vojvodová A. Rajcáni J, et al. Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
Cited by
- Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus.
Brack AR, Dijkstra JM, Granzow H, Klupp BG, Mettenleiter TC. Brack AR, et al. J Virol. 1999 Jul;73(7):5364-72. doi: 10.1128/JVI.73.7.5364-5372.1999. J Virol. 1999. PMID: 10364283 Free PMC article. - Epstein-Barr virus that lacks glycoprotein gN is impaired in assembly and infection.
Lake CM, Hutt-Fletcher LM. Lake CM, et al. J Virol. 2000 Dec;74(23):11162-72. doi: 10.1128/jvi.74.23.11162-11172.2000. J Virol. 2000. PMID: 11070013 Free PMC article. - The carboxy-terminal domain of glycoprotein N of human cytomegalovirus is required for virion morphogenesis.
Mach M, Osinski K, Kropff B, Schloetzer-Schrehardt U, Krzyzaniak M, Britt W. Mach M, et al. J Virol. 2007 May;81(10):5212-24. doi: 10.1128/JVI.01463-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229708 Free PMC article. - Glycoprotein M is an essential lytic replication protein of the murine gammaherpesvirus 68.
May JS, Colaco S, Stevenson PG. May JS, et al. J Virol. 2005 Mar;79(6):3459-67. doi: 10.1128/JVI.79.6.3459-3467.2005. J Virol. 2005. PMID: 15731240 Free PMC article. - The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: tracks, motors, and polymerization machines.
Bearer EL, Satpute-Krishnan P. Bearer EL, et al. Curr Drug Targets Infect Disord. 2002 Sep;2(3):247-64. doi: 10.2174/1568005023342407. Curr Drug Targets Infect Disord. 2002. PMID: 12462128 Free PMC article.
References
- Barnett B C, Dolan A, Telford E A R, Davison A J, McGeoch D J. A novel herpes simplex virus gene (UL49A) encodes a putative membrane protein with counterparts in other herpesviruses. J Gen Virol. 1992;73:2167–2171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous