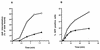Adenovirus internalization and infection require dynamin - PubMed (original) (raw)
Adenovirus internalization and infection require dynamin
K Wang et al. J Virol. 1998 Apr.
Abstract
The cell receptors that facilitate adenovirus internalization into cells have been identified; however, the infectious pathway of virus entry has not been established. Adenovirus entry and infection were examined in HeLa cells lacking or overexpressing mutant dynamin, a protein that specifically regulates clathrin-mediated endocytosis. Expression of mutant dynamin significantly reduced adenovirus internalization and gene delivery, indicating a functional requirement for this molecule. These findings are consistent with virus entry via the clathrin-coated pit pathway.
Figures
FIG. 1
Detection of mutant dynamin expression by immunoblotting. tTA-HeLa cells stably transfected with the K44A dynamin mutant were cultured in the presence (+ tet) or absence (− tet) of tetracycline for 48 h and then solubilized in sodium dodecyl sulfate sample buffer. Lysates prepared from 105 induced or uninduced cells were separated on a sodium dodecyl sulfate–7%-polyacrylamide gel under reducing conditions. Following transfer of the proteins to a nitrocellulose filter (Immobilon P; Amersham), the filter was probed with an antihemagglutinin epitope tag monoclonal antibody (12CA5) and then incubated with a goat anti-mouse immunoglobulin antibody conjugated to alkaline phosphatase. The blot was then developed by addition of a chromogenic substrate (Nitro Blue Tetrazolium).
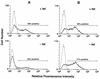
FIG. 2
Flow cytometric analysis of Ad-mediated gene delivery to control or mutant-dynamin-expressing HeLa cells. (A) tTA-HeLa cells were cultured in the presence (+ tet) or absence (− tet) of tetracycline for 48 h and then infected by incubation with Ad.RSV.GFP at a virus particle/cell ratio of 300 for 1 h at 37°C. The cells were then washed and recultured for 48 h in the presence or absence of tetracycline prior to flow cytometric analysis. Control cells (dotted lines) were incubated in medium without virus. (B) tTA-HeLa cells were cultured in the presence of tetracycline and then infected with Ad.RSV.GFP. The cells were then divided into two equal samples; one was cultured for 48 h in the presence of tetracycline (+ tet), and the other was cultured in the absence (− tet) of tetracycline. Both were then analyzed by flow cytometry.
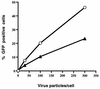
FIG. 3
Dose-dependent delivery of GFP to HeLa cells by use of recombinant Ad. tTA-HeLa cells were incubated in the presence (circles) or absence (triangles) of tetracycline for 48 h and then infected with Ad.RSV.GFP at various particle/cell ratios. GFP expression was analyzed 48 h later by flow cytometry. The data are representative of at least three experiments.
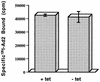
FIG. 4
Ad binding to HeLa cells expressing or lacking mutant dynamin. Cells were incubated for 48 h in the presence (+ tet) or absence (− tet) of tetracycline and then assayed for binding of 125I-labeled Ad type 2 (Ad2) particles as previously described (11). Nonspecific virus binding, which was subtracted from the total, was determined by incubating cells with a 200-fold excess of unlabeled virus particles. The data are the mean ± the standard deviation of triplicate samples.
FIG. 5
Ad internalization and gene delivery into HeLa cells expressing or lacking mutant dynamin. (A) Internalization of 125I-labeled Ad was measured in HeLa cells grown in the presence (circles) or absence (triangles) of tetracycline as previously described (25). Following warming of the cells to 37°C for various lengths of time, uninternalized virus particles were removed by incubating the cells in trypsin-EDTA and then washing them with HEPES-buffered saline. The data are the mean ± the standard deviation of triplicate samples. (B) In parallel studies, Ad-mediated gene delivery was examined in cells grown in the presence (circles) or absence (triangles) of tetracycline. Cells were incubated at 4°C with Ad.RSV.GFP at a particle/cell ratio of 300 and then warmed to 37°C for various lengths of time. After removal of uninternalized virus particles with trypsin-EDTA, the cells were cultured in the presence of tetracycline for 48 h prior to flow cytometric analysis. The data are representative of two experiments. Ad2, Ad type 2.
Similar articles
- Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway.
Lee S, Zhao Y, Anderson WF. Lee S, et al. J Virol. 1999 Jul;73(7):5994-6005. doi: 10.1128/JVI.73.7.5994-6005.1999. J Virol. 1999. PMID: 10364351 Free PMC article. - Dynamin is required for recombinant adeno-associated virus type 2 infection.
Duan D, Li Q, Kao AW, Yue Y, Pessin JE, Engelhardt JF. Duan D, et al. J Virol. 1999 Dec;73(12):10371-6. doi: 10.1128/JVI.73.12.10371-10376.1999. J Virol. 1999. PMID: 10559355 Free PMC article. - Induction of mutant dynamin specifically blocks endocytic coated vesicle formation.
Damke H, Baba T, Warnock DE, Schmid SL. Damke H, et al. J Cell Biol. 1994 Nov;127(4):915-34. doi: 10.1083/jcb.127.4.915. J Cell Biol. 1994. PMID: 7962076 Free PMC article. - Multiple pathways for the dynamin-regulated internalization of muscarinic acetylcholine receptors.
van Koppen CJ. van Koppen CJ. Biochem Soc Trans. 2001 Aug;29(Pt 4):505-8. doi: 10.1042/bst0290505. Biochem Soc Trans. 2001. PMID: 11498018 Review. - Use of green fluorescent protein (GFP) to study cellular dynamics. Constructing GFP-tagged motor enzymes.
Cao H, Thompson H, Krueger EW, McNiven M. Cao H, et al. Methods Mol Biol. 2001;161:165-87. doi: 10.1385/1-59259-051-9:165. Methods Mol Biol. 2001. PMID: 11190504 Review. No abstract available.
Cited by
- Inhibition of betanodavirus infection by inhibitors of endosomal acidification.
Adachi K, Ichinose T, Takizawa N, Watanabe K, Kitazato K, Kobayashi N. Adachi K, et al. Arch Virol. 2007;152(12):2217-24. doi: 10.1007/s00705-007-1061-7. Epub 2007 Sep 22. Arch Virol. 2007. PMID: 17891330 Free PMC article. - Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors.
Bartlett JS, Wilcher R, Samulski RJ. Bartlett JS, et al. J Virol. 2000 Mar;74(6):2777-85. doi: 10.1128/jvi.74.6.2777-2785.2000. J Virol. 2000. PMID: 10684294 Free PMC article. - The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol.
Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. Nakano MY, et al. J Virol. 2000 Aug;74(15):7085-95. doi: 10.1128/jvi.74.15.7085-7095.2000. J Virol. 2000. PMID: 10888649 Free PMC article. - Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35.
Kälin S, Amstutz B, Gastaldelli M, Wolfrum N, Boucke K, Havenga M, DiGennaro F, Liska N, Hemmi S, Greber UF. Kälin S, et al. J Virol. 2010 May;84(10):5336-50. doi: 10.1128/JVI.02494-09. Epub 2010 Mar 17. J Virol. 2010. PMID: 20237079 Free PMC article. - Early steps of clathrin-mediated endocytosis involved in phagosomal escape of Fcgamma receptor-targeted adenovirus.
Meier O, Gastaldelli M, Boucke K, Hemmi S, Greber UF. Meier O, et al. J Virol. 2005 Feb;79(4):2604-13. doi: 10.1128/JVI.79.4.2604-2613.2005. J Virol. 2005. PMID: 15681460 Free PMC article.
References
- Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
- Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. - PubMed
- Fitzgerald D J P, Padmanabhan R, Pastan I, Willingham M C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983;32:607–617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources