Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons - PubMed (original) (raw)
Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons
R J Crowder et al. J Neurosci. 1998.
Abstract
Recent studies have suggested a role for phosphatidylinositol (PI) 3-kinase in cell survival, including the survival of neurons. We used rat sympathetic neurons maintained in vitro to characterize the potential survival signals mediated by PI 3-kinase and to test whether the Akt protein kinase, a putative effector of PI 3-kinase, functions during nerve growth factor (NGF)-mediated survival. Two PI 3-kinase inhibitors, LY294002 and wortmannin, block NGF-mediated survival of sympathetic neurons. Cell death caused by LY294002 resembles death caused by NGF deprivation in that it is blocked by a caspase inhibitor or a cAMP analog and that it is accompanied by the induction of c-jun, c-fos, and cyclin D1 mRNAs. Treatment of neurons with NGF activates endogenous Akt protein kinase, and LY294002 or wortmannin blocks this activation. Expression of constitutively active Akt or PI 3-kinase in neurons efficiently prevents death after NGF withdrawal. Conversely, expression of dominant negative forms of PI 3-kinase or Akt induces apoptosis in the presence of NGF. These results demonstrate that PI 3-kinase and Akt are both necessary and sufficient for the survival of NGF-dependent sympathetic neurons.
Figures
Fig. 1.
LY294002 kills sympathetic neurons in a manner that morphologically resembles NGF deprivation. The 5 d neuronal cultures were treated with or without 100 μ
m
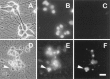
LY294002 in AM50 media. Approximately 72 hr later the neurons were fixed, processed for TUNEL analysis and Hoechst staining, and photographed.A–C show phase-contrast, Hoechst-stained chromatin, and TUNEL-labeled views, respectively, from the same field of NGF-maintained (nontreated) control cultures. D–F show parallel views of LY294002-treated neurons. _Arrowheads_point to a representative apoptotic neuron with a degraded soma containing a condensed, fragmented nucleus labeled by TUNEL. Scale bar, 30 μm.
Fig. 2.
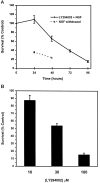
Death of sympathetic neurons by LY294002 is time- and dose-dependent. A, The 5 d neuronal cultures were treated with 100 μ
m
LY294002 in AM50 or were deprived of NGF. Survival was assayed at 24, 48, 72, and 96 hr after a single application of LY294002 or after 24 and 48 hr of NGF deprivation by counting Nissl-stained neurons. Results represent mean ± SEM from three independent experiments. B, The 5 d cultures were treated with a single dose of 10, 30, or 100 μ
m
LY294002 in AM50. Survival was assayed after 4 d by counting Nissl-stained neurons, as described in Materials and Methods. The 100 μ
m
LY294002 treatment data are the same as those shown in A. Results represent the mean ± SEM from three independent experiments and are presented as a percentage of the survival in control NGF-maintained cultures.
Fig. 3.
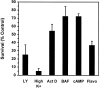
BAF, cpt-cAMP, and actinomycin D inhibit LY294002-induced death. The 5 d cultures maintained in AM50 were treated with 100 μ
m
LY294002 alone (LY) or in the presence of each of the following: 1 μ
m
flavopiridol (Flavo), 0.1 μg/ml actinomycin D (ActD), 100 μ
m
BAF (BAF), or 300 μ
m
cpt-cAMP (cAMP). For one set of treatments the neurons maintained in depolarizing concentrations of potassium without NGF were treated with 100 μ
m
LY294002 (High K+). Neuronal survival was assayed after 80 hr by counting Nissl-stained neurons. In control experiments each agent effectively prevented the death of NGF-deprived neurons (data not shown). Results represent the mean ± range from two independent experiments and are presented as a percentage of the survival in control NGF-maintained cultures.
Fig. 4.
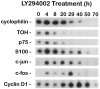
Expression of cyclin D1,c-jun, and c-fos increase during LY294002-induced death. Neurons cultured in AM50 for 5 d received no treatment (0 hr) or were treated with 100 μ
m
LY294002 in AM50 for the indicated time intervals. Relative changes in the mRNA levels of specific genes were measured by semiquantitative RT-PCR analysis, as outlined in Materials and Methods. Shown are representative results from one of three independent time courses, all of which yielded similar results. PCR cycle numbers for each of the genes were as follows: cyclophilin, 18 cycles; tyrosine hydroxylase (TOH), 18 cycles; p75 neurotrophin receptor (p75), 18 cycles; c-fos, 24 cycles; c-jun, 24 cycles; cyclin D1, 25 cycles; S100β, 28 cycles.
Fig. 5.
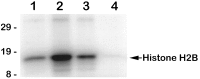
NGF stimulates Akt protein kinase activity in a PI 3-kinase-dependent manner. Akt protein kinase activity was analyzed in immune complex kinase assays prepared from SCG neurons treated as follows: lane 1, neurons deprived of NGF for 10 hr;lane 2, neurons deprived of NGF for 10 hr and then stimulated with NGF for 15 min; lane 3, neurons deprived of NGF for 10 hr and then treated with NGF for 15 min in the presence of 100 μ
m
LY294002; lane 4, mock kinase reaction containing only histone H2B, [γ-32P]ATP, and protein A beads. Reaction products were analyzed by SDS-PAGE, followed by autoradiography and PhosphorImager analysis. Akt protein kinase activity increased an average of 3.1-fold after NGF stimulation (n = 4).
Fig. 6.
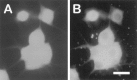
Constitutively active PI 3-kinase (p110*) is expressed efficiently in microinjected sympathetic neurons. Neurons were injected with p110* plasmid and lysine-fixable tetramethylrhodamine-dextran. At 15 hr after microinjection the neurons were fixed and stained with the 9E10 monoclonal antibody, which recognizes the myc epitope attached to p110*. The 9E10 antibody was detected by FITC-conjugated anti-mouse antibodies. A_shows rhodamine-labeled (injected) cells. B, Immunofluorescence analysis shows that all of the injected neurons in_A overexpress p110*. Overall, p110* was detected in 90% of the injected neurons. Scale bar, 30 μm.
Fig. 7.
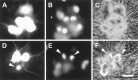
Overexpression of constitutively active PI 3-kinase is sufficient to promote the survival of NGF-deprived sympathetic neurons. Shown are photomicrographs of NGF-deprived neurons injected with p110* (A–C) or p110*Δkin (D–F) expression vectors. Neurons were microinjected with the appropriate expression vectors, allowed to recover in AM50 for 12–15 hr, and then deprived of NGF for 48 hr. Neurons were stained with Hoechst dye and photographed. Photographs show rhodamine (injected cells) (A, D), Hoechst-stained nuclei (B, E), and phase-contrast views (C, F) of one field of cells for each injected DNA. Cells scored as alive in these experiments retained spherical, phase-bright cell bodies, intact neurites, and uniformly dispersed chromatin (A–C). Dead cells were characterized by phase-dark somas, fragmented neurites, and nuclear remnants devoid of stained chromatin or nuclei containing highly condensed chromatin (D–F). The arrowhead in_D_ points to the remnant of a cell devoid of a nucleus. The arrowheads in E and _F_indicate phase-dark cells containing condensed chromatin. Scale bar, 40 μm.
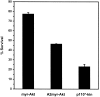
Fig. 8.
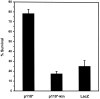
Quantitation of neuronal survival mediated by constitutively active PI 3-kinase. Neurons cultured in AM50 for 5–6 d were microinjected with expression vectors for p110*, p110*Δkin, and LacZ and then deprived of NGF for 48 hr. Survival was assayed by a blinded observer in accordance with the criteria described above. Error bars represent the mean ± SEM from four independent experiments. Survival for each of the following injections was p110*, 78.3 ± 4.3%; p110*Δkin, 17.3 ± 2.5%; and LacZ, 25.7 ± 5.9%. Survival with p110*-injected cells was significantly greater than p110*Δkin-injected or LacZ-injected cells (two-tailed_p_ values = 0.0001 and 0.0003, respectively). Survival between p110*Δkin and LacZ was not significantly different (p > 0.35).
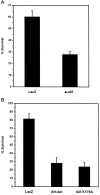
Fig. 9.
Overexpression of activated Akt (myr-Akt) is sufficient to promote the survival of NGF-deprived sympathetic neurons. Neurons cultured in AM50 for 5–6 d were microinjected with myr-Akt, A2myr-Akt, or p110*Δkin (used as a negative control) expression vectors and then deprived of NGF for ∼48 hr. Then the injected cells were evaluated for survival. Error bars represent the mean ± SEM from three independent experiments. Survival for each of the following injections was myr-Akt, 77.4 ± 1.3%; A2myr-Akt, 46.5 ± 0.26%; and p110*Δkin, 23.2 ± 2.4%. Survival with myr-Akt-injected cells was significantly greater than for both A2myr-Akt-injected and p110*Δkin-injected cells (two-tailed p values = 2.1 × 10−5 and 3.8 × 10−5, respectively). Survival between A2myr-Akt and p110*Δkin was also significantly different (p = 0.0006).
Fig. 10.
Expression of dominant negative PI 3-kinase (Δp85), kinase-inactive Akt (AktK179A), or a truncated dominant negative Akt (AH-Akt) inhibits NGF-mediated neuronal survival. Neurons cultured in AM50 for 5–6 d were microinjected with LacZ or Δp85 (A) or LacZ, AH-Akt·Flag, or AktK179A expression vectors (B) and then maintained in AM50 media for 3 d. Then the injected cells were evaluated for survival.A, Survival was 60.2 ± 5.2% and 27.9 ± 2.5% for LacZ and Δp85, respectively (two-tailed _p_value = 0.001). Error bars represent the mean ± SEM from four independent experiments. B, Survival for the following injections were LacZ, 81.6 ± 6.3%; AH-Akt, 28.4 ± 6.7%; and AktK179A, 24.1 ± 4.9%. Error bars represent the mean ± SEM from four experiments for LacZ and AH-Akt and three experiments for AktK179A. Survival of AH-Akt-injected or AktK179A-injected neurons was significantly less than the survival of LacZ-injected cells (two-tailed p value = 0.001). Survival between AH-Akt and AktK179A was not significantly different (p = 0.65).
Similar articles
- Role of PI 3-kinase, Akt and Bcl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons.
Orike N, Middleton G, Borthwick E, Buchman V, Cowen T, Davies AM. Orike N, et al. J Cell Biol. 2001 Sep 3;154(5):995-1005. doi: 10.1083/jcb.200101068. Epub 2001 Aug 27. J Cell Biol. 2001. PMID: 11524433 Free PMC article. - Nerve growth factor-induced PKB/Akt activity is sustained by phosphoinositide 3-kinase dependent and independent signals in sympathetic neurons.
Virdee K, Xue L, Hemmings BA, Goemans C, Heumann R, Tolkovsky AM. Virdee K, et al. Brain Res. 1999 Aug 7;837(1-2):127-42. doi: 10.1016/s0006-8993(99)01643-1. Brain Res. 1999. PMID: 10433995 - PI 3-kinase, Akt and cell survival.
Downward J. Downward J. Semin Cell Dev Biol. 2004 Apr;15(2):177-82. doi: 10.1016/j.semcdb.2004.01.002. Semin Cell Dev Biol. 2004. PMID: 15209377 Review. - Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival.
Matsui T, Nagoshi T, Rosenzweig A. Matsui T, et al. Cell Cycle. 2003 May-Jun;2(3):220-3. Cell Cycle. 2003. PMID: 12734428 Review.
Cited by
- The JNK- and AKT/GSK3β- signaling pathways converge to regulate Puma induction and neuronal apoptosis induced by trophic factor deprivation.
Ambacher KK, Pitzul KB, Karajgikar M, Hamilton A, Ferguson SS, Cregan SP. Ambacher KK, et al. PLoS One. 2012;7(10):e46885. doi: 10.1371/journal.pone.0046885. Epub 2012 Oct 3. PLoS One. 2012. PMID: 23056511 Free PMC article. - The invulnerability of adult neurons: a critical role for p73.
Walsh GS, Orike N, Kaplan DR, Miller FD. Walsh GS, et al. J Neurosci. 2004 Oct 27;24(43):9638-47. doi: 10.1523/JNEUROSCI.1299-04.2004. J Neurosci. 2004. PMID: 15509751 Free PMC article. - Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal.
Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Hetman M, et al. J Neurosci. 2000 Apr 1;20(7):2567-74. doi: 10.1523/JNEUROSCI.20-07-02567.2000. J Neurosci. 2000. PMID: 10729337 Free PMC article. - Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival.
Meucci O, Fatatis A, Simen AA, Miller RJ. Meucci O, et al. Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):8075-80. doi: 10.1073/pnas.090017497. Proc Natl Acad Sci U S A. 2000. PMID: 10869418 Free PMC article. - Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival.
Blair LA, Bence-Hanulec KK, Mehta S, Franke T, Kaplan D, Marshall J. Blair LA, et al. J Neurosci. 1999 Mar 15;19(6):1940-51. doi: 10.1523/JNEUROSCI.19-06-01940.1999. J Neurosci. 1999. PMID: 10066247 Free PMC article.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B-alpha. Curr Biol. 1997;7:261–269. - PubMed
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
- Borasio GD, John J, Wittinghofer A, Barde YA, Sendtner M, Heumann R. Ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurons. Neuron. 1989;2:1087–1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous