Snt309p, a component of the Prp19p-associated complex that interacts with Prp19p and associates with the spliceosome simultaneously with or immediately after dissociation of U4 in the same manner as Prp19p - PubMed (original) (raw)
Snt309p, a component of the Prp19p-associated complex that interacts with Prp19p and associates with the spliceosome simultaneously with or immediately after dissociation of U4 in the same manner as Prp19p
H R Chen et al. Mol Cell Biol. 1998 Apr.
Free PMC article
Abstract
The yeast protein Prp19p is essential for pre-mRNA splicing and is associated with the spliceosome concurrently with or just after dissociation of U4 small nuclear RNA. In splicing extracts, Prp19p is associated with several other proteins in a large protein complex of unknown function, but at least one of these proteins is also essential for splicing (W.-Y. Tarn, C.-H. Hsu, K.-T. Huang, H.-R. Chen, H.-Y. Kao, K.-R. Lee, and S.-C. Cheng, EMBO J. 13:2421-2431, 1994). To identify proteins in the Prp19p-associated complex, we have isolated trans-acting mutations that exacerbate the phenotypes of conditional alleles of prp19, using the ade2-ade3 sectoring system. A novel splicing factor, Snt309p, was identified through such a screen. Although the SNT309 gene was not essential for growth of Saccharomyces cerevisiae under normal conditions, yeast cells containing a null allele of the SNT309 gene were temperature sensitive and accumulated pre-mRNA at the nonpermissive temperature. Far-Western blot analysis revealed direct interaction between Prp19p and Snt309p. Snt309p was shown to be a component of the Prp19p-associated complex by Western blot analysis. Immunoprecipitation studies demonstrated that Snt309p was also a spliceosomal component and associated with the spliceosome in the same manner as Prp19p during spliceosome assembly. These results suggest that the functions of Prp19p and Snt309p in splicing may require coordinate action of these two proteins.
Figures
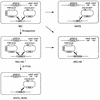
FIG. 1
Scheme for screen of mutants synthetic lethal to_prp19_ temperature-sensitive mutants.
FIG. 2
Nucleotide and encoded protein sequences of the_SNT309_ gene. The gene encodes a protein of 175 amino acid residues. No identifiable motif is found in the protein sequence. Neither the nucleotide nor the protein sequence has homology to an existing sequence in the database.
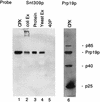
FIG. 3
Snt309p can interact with Prp19p in far-Western blots. Prp19p isolated as a protein complex (lane 1), expressed in_E. coli_ (lane 2), as a purified protein (lane 3), in yeast extract from an overproducing strain (lane 4), and in a 40P fraction from a regular strain (lane 5) probed with in vitro-translated, 35S-labeled Snt309p protein in far-Western blots and the Prp19p-associated complex probed with labeled Prp19p (lane 6) are shown. Lanes 2, 4, and 5 each contain approximately 100 μg of total proteins. CPX, complex; Ex, extract.
FIG. 4
Disruption of the SNT309 gene results in a temperature-sensitive phenotype. A diploid strain carrying one copy of the wild-type and one copy of the LEU2_-disrupted_SNT309 gene was sporulated for tetrad analysis. Shown are nine tetrads after transfer to a fresh YPD plate and growth at 25°C. They were then replica plated to test for leucine auxotrophy and growth phenotype at various temperatures.
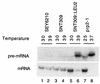
FIG. 5
The _SNT309_-disrupted yeast strain accumulates pre-mRNA in vivo. The SEY6210, _SNT309_-nondisrupted, and_SNT309_-disrupted strains isolated from the same tetrad were grown at 30°C until mid-log phase and then shifted to 39°C and grown for 2 h. RNA was extracted from cells grown at both 30 and 39°C and hybridized with an actin intron probe to reveal the pre-mRNA or with an actin message probe. The prp2-1 mutant was grown at 25°C and then shifted to 37°C. Each lane contained 10 μg of total RNA.
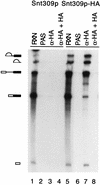
FIG. 6
The Snt309p protein is associated with the spliceosome during the splicing reaction. Mixtures of the splicing reactions (20 μl) carried out in the extract prepared from the wild-type and Snt309p HA-tagged strains (lanes 1 and 5) were precipitated with the anti-HA antibody (lanes 3 and 7) or without the antibody (lanes 2 and 6). The antibody was also preincubated with excessive amounts of the HA peptide before immunoprecipitation (lanes 4 and 8). The pre-mRNA, E1, IVS, and IVS-E2, but only very small amounts of the mature message, were precipitated in the HA-tagged extract. Lanes 1 and 5 contain 2 μl of the splicing reaction mixtures. Diagrams on the left correspond to the structures in Fig. 7A. α-HA, anti-HA antibody; RXN, 1 to 2 μl of the splicing reaction mixture; PAS, protein A-Sepharose.
FIG. 7
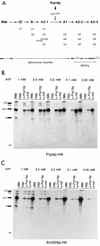
Snt309p is associated with the spliceosome in the same manner as Prp19p. (A) A scheme for spliceosome assembly showing that Prp19p becomes associated with the spliceosome during transition from complex A2-1 to A1, which can be blocked at lower ATP concentrations. Also indicated is Prp4p, which is tightly associated with the U4 snRNP and is present only in complex A2-1 during spliceosome assembly. (B and C) Splicing reactions (20-μl mixtures) carried out at various ATP concentrations in Prp4p HA-tagged or Snt309p HA-tagged extracts, respectively, were precipitated with anti-Prp19p antibody and anti-HA antibody. RXN, 1 to 2 μl of the splicing reaction mixture; PAS, protein A-Sepharose; α-Prp19p, anti-Prp19p antibody; α-HA, anti-HA antibody. Symbols: □, 5′-exon; ▪, 3′-exon; , lariot intron.
, lariot intron.
FIG. 8
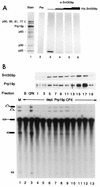
Immunoblot analysis showing that Snt309p is a component of the Prp19p-associated complex. (A) The Prp19p-associated complex was analyzed for the presence of Snt309p by immunoblot analysis using anti-Snt309p antibody (lanes 3 to 6) or preimmune serum (lane 2). The antibody was preincubated with increasing amounts of recombinant Snt309p to block the reaction (lanes 4 to 6). Lane 1, components of the Prp19p-associated complex revealed by silver staining. (B) Immunoblot analysis using anti-Snt309p and anti-Prp19p antibodies and complementation of the Prp19p-depleted extract of gradient fractions. Lane M, mock-treated extract; CPX, complementation with the Prp19p-associated complex.
Similar articles
- Snt309p modulates interactions of Prp19p with its associated components to stabilize the Prp19p-associated complex essential for pre-mRNA splicing.
Chen HR, Tsao TY, Chen CH, Tsai WY, Her LS, Hsu MM, Cheng SC. Chen HR, et al. Proc Natl Acad Sci U S A. 1999 May 11;96(10):5406-11. doi: 10.1073/pnas.96.10.5406. Proc Natl Acad Sci U S A. 1999. PMID: 10318896 Free PMC article. - Identification and characterization of two novel components of the Prp19p-associated complex, Ntc30p and Ntc20p.
Chen CH, Tsai WY, Chen HR, Wang CH, Cheng SC. Chen CH, et al. J Biol Chem. 2001 Jan 5;276(1):488-94. doi: 10.1074/jbc.M006958200. J Biol Chem. 2001. PMID: 11018040 - Cef1p is a component of the Prp19p-associated complex and essential for pre-mRNA splicing.
Tsai WY, Chow YT, Chen HR, Huang KT, Hong RI, Jan SP, Kuo NY, Tsao TY, Chen CH, Cheng SC. Tsai WY, et al. J Biol Chem. 1999 Apr 2;274(14):9455-62. doi: 10.1074/jbc.274.14.9455. J Biol Chem. 1999. PMID: 10092627 - Structural dynamics of the N-terminal domain and the Switch loop of Prp8 during spliceosome assembly and activation.
Jia X, Sun C. Jia X, et al. Nucleic Acids Res. 2018 May 4;46(8):3833-3840. doi: 10.1093/nar/gky242. Nucleic Acids Res. 2018. PMID: 29635373 Free PMC article. Review. - Multiple genetic and biochemical interactions of Brr2, Prp8, Prp31, Prp1 and Prp4 kinase suggest a function in the control of the activation of spliceosomes in Schizosaccharomyces pombe.
Bottner CA, Schmidt H, Vogel S, Michele M, Käufer NF. Bottner CA, et al. Curr Genet. 2005 Sep;48(3):151-61. doi: 10.1007/s00294-005-0013-6. Epub 2005 Oct 12. Curr Genet. 2005. PMID: 16133344 Review.
Cited by
- Functional and physical interactions between components of the Prp19p-associated complex.
Chen CH, Yu WC, Tsao TY, Wang LY, Chen HR, Lin JY, Tsai WY, Cheng SC. Chen CH, et al. Nucleic Acids Res. 2002 Feb 15;30(4):1029-37. doi: 10.1093/nar/30.4.1029. Nucleic Acids Res. 2002. PMID: 11842115 Free PMC article. - Yeast and human genes that affect the Escherichia coli SOS response.
Perkins EL, Sterling JF, Hashem VI, Resnick MA. Perkins EL, et al. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2204-9. doi: 10.1073/pnas.96.5.2204. Proc Natl Acad Sci U S A. 1999. PMID: 10051619 Free PMC article. - Cwc25 is a novel splicing factor required after Prp2 and Yju2 to facilitate the first catalytic reaction.
Chiu YF, Liu YC, Chiang TW, Yeh TC, Tseng CK, Wu NY, Cheng SC. Chiu YF, et al. Mol Cell Biol. 2009 Nov;29(21):5671-8. doi: 10.1128/MCB.00773-09. Epub 2009 Aug 24. Mol Cell Biol. 2009. PMID: 19704000 Free PMC article. - Functional links between the Prp19-associated complex, U4/U6 biogenesis, and spliceosome recycling.
Chen CH, Kao DI, Chan SP, Kao TC, Lin JY, Cheng SC. Chen CH, et al. RNA. 2006 May;12(5):765-74. doi: 10.1261/rna.2292106. Epub 2006 Mar 15. RNA. 2006. PMID: 16540691 Free PMC article. - Genetic analysis reveals a role for the C terminus of the Saccharomyces cerevisiae GTPase Snu114 during spliceosome activation.
Brenner TJ, Guthrie C. Brenner TJ, et al. Genetics. 2005 Jul;170(3):1063-80. doi: 10.1534/genetics.105.042044. Epub 2005 May 23. Genetics. 2005. PMID: 15911574 Free PMC article.
References
- Abovich N, Liao X C, Rosbash M. The yeast MUD2 protein: an interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes Dev. 1994;8:843–854. - PubMed
- Beggs J D. Yeast protein splicing factors involved in nuclear pre-mRNA splicing. Mol Biol Rep. 1993;18:99–103. - PubMed
- Behrens S-E, Galisson F, Legrain P, Lührmann R. Evidence that the 60-kDa protein of 17S U2 small nuclear ribonucleoprotein is immunologically and functionally related to the yeast PRP9 splicing factor and is required for the efficient formation of prespliceosomes. Proc Natl Acad Sci USA. 1993;90:8229–8233. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous