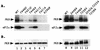Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2 - PubMed (original) (raw)
Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2
P R Romano et al. Mol Cell Biol. 1998 Apr.
Abstract
The human double-stranded RNA-dependent protein kinase (PKR) is an important component of the interferon response to virus infection. The activation of PKR is accompanied by autophosphorylation at multiple sites, including one in the N-terminal regulatory region (Thr-258) that is required for full kinase activity. Several protein kinases are activated by phosphorylation in the region between kinase subdomains VII and VIII, referred to as the activation loop. We show that Thr-446 and Thr-451 in the PKR activation loop are required in vivo and in vitro for high-level kinase activity. Mutation of either residue to Ala impaired translational control by PKR in yeast cells and COS1 cells and led to tumor formation in mice. These mutations also impaired autophosphorylation and eukaryotic initiation factor 2 subunit alpha (eIF2alpha) phosphorylation by PKR in vitro. Whereas the Ala-446 substitution substantially reduced PKR function, the mutant kinase containing Ala-451 was completely inactive. PKR specifically phosphorylated Thr-446 and Thr-451 in synthetic peptides in vitro, and mass spectrometry analysis of PKR phosphopeptides confirmed that Thr-446 is an autophosphorylation site in vivo. Substitution of Glu-490 in subdomain X of PKR partially restored kinase activity when combined with the Ala-451 mutation. This finding suggests that the interaction between subdomain X and the activation loop, described previously for MAP kinase, is a regulatory feature conserved in PKR. We found that the yeast eIF2alpha kinase GCN2 autophosphorylates at Thr-882 and Thr-887, located in the activation loop at exactly the same positions as Thr-446 and Thr-451 in PKR. Thr-887 was more critically required than was Thr-882 for GCN2 kinase activity, paralleling the relative importance of Thr-446 and Thr-451 in PKR. These results indicate striking similarities between GCN2 and PKR in the importance of autophosphorylation and the conserved Thr residues in the activation loop.
Figures
FIG. 1
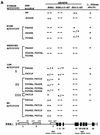
Established phosphorylation sites in the activation loops of several protein kinases. Sequence alignments of the segments between kinase subdomains VII and VIII are shown for the protein kinases listed on the left (19, 40). Highly conserved residues common to all of the kinases are shown in bold type without underlining. Phosphorylated residues required for activation of the kinases are shown in bold type and underlined. The kinases were Erk2 (extracellular signal-regulated kinase), Cdk2 (cyclin-dependent kinase), PKA (cyclic AMP-dependent protein kinase), RSK2 (ribosomal protein S6 kinase), GSK3α (glycogen synthase kinase), dGCN2 (Drosophila GCN2 homolog), and HRI (heme-regulated inhibitor). Potential autophosphorylation sites examined in the PKR and GCN2 activation loops are indicated (positions 446, 448, and 451 and positions 882 and 887, respectively). The identification of Thr-446 in PKR and Thr-882 and Thr-887 in GCN2 as autophosphorylation sites is the subject of this report.
FIG. 2
In vivo analysis of mutant PKR alleles by growth tests in yeast. (A) The schematic at the bottom represents the full-length wild-type PKR protein sequence. The boxes in the amino-terminal half represent the three regions rich in basic residues. Boxes 1 and 2 contain DRBM-1 and -2, respectively. Box 3 is a region rich in basic amino acids of unknown function. The amino acid residues spanning these regions are indicated. The black boxes in the C-terminal half (I to XI) represent conserved kinase subdomains found in the catalytic regions of all protein kinases. The locations of known (S242, T255, and T258) or suspected (T446, S448, and T451) autophosphorylation sites are indicated. Plasmids carrying the indicated PKR alleles were introduced into the _gcn2_Δ yeast strain H1816 expressing wild-type eIF2α. Patches of transformants were grown to confluence in SD medium and replica plated to SD medium plus 30 mM 3-AT, SGAL medium, and SGAL medium plus 30 mM 3-AT. Plates were incubated for 3 to 4 days at 30°C, and growth was scored relative to that of the wild-type (WT) PKR transformants. Relative growth from strongest to weakest was scored as ++, +, +/−, −/+, and −−. Whole-cell lysates from the indicated strains that were either treated or not treated with λ protein phosphatase (λ PPase) were subjected to SDS-PAGE fractionation and immunoblot analysis as described in the legends to Fig. 3 and 4. PKR proteins whose mobilities increased when treated with phosphatase are indicated by +; proteins that showed no discernible change in mobility when treated with phosphatase are indicated by −. (B) Selected transformants of H1816 bearing the indicated PKR constructs were streaked on SGAL medium and incubated for 6 days at 30°C.
FIG. 2
In vivo analysis of mutant PKR alleles by growth tests in yeast. (A) The schematic at the bottom represents the full-length wild-type PKR protein sequence. The boxes in the amino-terminal half represent the three regions rich in basic residues. Boxes 1 and 2 contain DRBM-1 and -2, respectively. Box 3 is a region rich in basic amino acids of unknown function. The amino acid residues spanning these regions are indicated. The black boxes in the C-terminal half (I to XI) represent conserved kinase subdomains found in the catalytic regions of all protein kinases. The locations of known (S242, T255, and T258) or suspected (T446, S448, and T451) autophosphorylation sites are indicated. Plasmids carrying the indicated PKR alleles were introduced into the _gcn2_Δ yeast strain H1816 expressing wild-type eIF2α. Patches of transformants were grown to confluence in SD medium and replica plated to SD medium plus 30 mM 3-AT, SGAL medium, and SGAL medium plus 30 mM 3-AT. Plates were incubated for 3 to 4 days at 30°C, and growth was scored relative to that of the wild-type (WT) PKR transformants. Relative growth from strongest to weakest was scored as ++, +, +/−, −/+, and −−. Whole-cell lysates from the indicated strains that were either treated or not treated with λ protein phosphatase (λ PPase) were subjected to SDS-PAGE fractionation and immunoblot analysis as described in the legends to Fig. 3 and 4. PKR proteins whose mobilities increased when treated with phosphatase are indicated by +; proteins that showed no discernible change in mobility when treated with phosphatase are indicated by −. (B) Selected transformants of H1816 bearing the indicated PKR constructs were streaked on SGAL medium and incubated for 6 days at 30°C.
FIG. 3
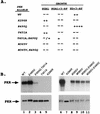
Immunoblot analysis of PKR protein levels in _gcn2_Δ yeast cells expressing wild-type eIF2α. Transformants of yeast strain H1816 bearing the indicated wild-type (WT) or mutant PKR alleles were grown in SD medium at 30°C for 30 h and then shifted to SGAL medium for 12 h to induce PKR expression. Thirty micrograms of total cell protein from each strain was fractionated by SDS-PAGE with 10% polyacrylamide gels and subjected to immunoblot analysis with monoclonal antibodies against PKR (71/10) and monoclonal antibodies against PAB1 (PAB), the yeast poly(A)-binding protein. The latter were used to confirm that equal amounts of cell protein were analyzed for each strain. ECL was used to visualize immune complexes. Lanes 10 and 11, 12 and 13, and 14 and 15 contain extracts isolated from two independent transformants bearing the indicated PKR alleles.
FIG. 4
Immunoblot analysis of protein levels and relative mobilities of PKR proteins expressed in yeast cells containing nonphosphorylatable eIF2α-S51A. (A and B) Thirty micrograms of total cell protein from yeast strain H1817 expressing wild-type (WT) PKR protein or the indicated PKR mutant proteins was subjected to SDS-PAGE with 10% (A) or 7.5% (B) polyacrylamide gels and immunoblot analysis with PKR-specific monoclonal antiserum and ECL to detect immune complexes. (C) Comparison of the relative mobilities of PKR proteins with and without phosphatase treatment. Thirty micrograms of total cell protein from transformants of the H1817 strain were treated with λ protein phosphatase (+ λ PPase). Phosphatase-treated and untreated samples were subjected to SDS-PAGE (7.5% polyacrylamide gel) and immunoblot analysis. The broken lines in panels B and C are drawn midway through the K296R PKR bands. The displacements of the midpoints of the untreated wild-type and T451S PKR bands from these lines were 2.7 and 2.0 mm, respectively, whereas the protein bands of the phosphatase-treated samples were displaced from these lines by 1.2 and 0.5 mm, respectively.
FIG. 5
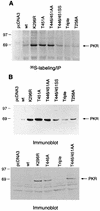
In vivo autophosphorylation of wild-type and mutant PKR proteins in yeast cells containing eIF2α-S51A. Transformants of yeast strain H1817 (containing nonphosphorylatable eIF2α-S51A) bearing the indicated wild-type (WT) or mutant PKR alleles were grown in SGAL medium to induce PKR expression and metabolically labeled with [32P]orthophosphate for 4 to 6 h (see Materials and Methods). PKR proteins were immunoprecipitated from aliquots of labeled whole-cell extracts with polyclonal antibodies against PKR, and the immune complexes were analyzed by SDS-PAGE (10% polyacrylamide) followed by autoradiography.
FIG. 6
In vitro kinase activities of immunopurified PKR proteins isolated from yeast strains containing eIF2α-S51A. (A) Transformants of strain H1817 bearing the indicated wild-type (WT) or mutant PKR alleles were grown in SGAL medium to induce PKR expression, and the PKR proteins were immunoprecipitated from whole-cell extracts containing 150 μg of total protein with polyclonal antibodies against PKR. Immune complexes were incubated in kinase reaction buffer (KRB) (38) in the presence of [γ-32P]ATP and 1 μg of purified recombinant eIF2α for 15 min at 30°C. Radiolabeled samples were separated by SDS-PAGE (8 to 16% polyacrylamide gradient gel) and transferred to a nitrocellulose filter. The radiolabeled PKR and eIF2α proteins were visualized by autoradiography. It was shown previously that PKR immunopurified from yeast extracts was activated without the addition of dsRNA activators and that the addition of poly(I) · poly(C) produced no significant increase in activity for either mutant or wild-type enzymes (38); therefore, these assays were conducted without the addition of exogenous dsRNA. (B) The same nitrocellulose filter was subjected to immunoblot analysis with monoclonal antibodies against PKR as described in the legends to Fig. 3 and 4 to visualize the amounts of PKR immunoprecipitated for each sample.
FIG. 7
Substitution of Glu-490 partially suppresses the impairment of kinase activity imposed by the T451A mutation in the PKR activation loop in vivo and in vitro. (A) In vivo analysis. Plasmids carrying the indicated PKR alleles were introduced into the _gcn2_Δ yeast strain H1816 expressing wild-type eIF2α. Patches of transformants were grown to confluence in SD medium and replica plated to the indicated media. Growth was scored relative to that of the strain containing wild-type (WT) PKR as described in the legend to Fig. 2. (B) In vitro analysis. Transformants of strain H1817 (expressing eIF22-S51A) bearing the indicated wild-type (WT) or mutant PKR alleles were grown in SGAL medium to induce PKR expression, and the PKR proteins were immunoprecipitated from whole-cell extracts containing 150 μg of total protein with polyclonal antibodies against PKR. In vitro kinase assays were carried out as described in Materials and Methods. Radiolabeled samples were separated by SDS-PAGE (10% polyacrylamide gel) and transferred to a nitrocellulose filter. The radiolabeled PKR proteins were visualized by autoradiography (upper panel). The same nitrocellulose filter was subjected to immunoblot analysis with monoclonal antibodies against PKR to visualize the amounts of PKR immunoprecipitated for each sample (lower panel).
FIG. 8
Analysis of autoregulation of PKR expression in mammalian cells. The indicated PKR alleles under the control of a CMV promoter were transfected into COS1 cells (see Materials and Methods), and the cells were harvested 48 h postinfection. (A) Transfected cells were labeled with [35S]methionine, and the PKR proteins were immunoprecipitated (IP) from 500 μg of total cytoplasmic protein with anti-PKR polyclonal antibodies. Radiolabeled samples were resolved by SDS-PAGE followed by autoradiography. wt, wild type. (B) Immunoblot analysis of PKR expression levels in COS1 cells. Total cytoplasmic protein (75 μg) was subjected to SDS-PAGE followed by immunoblot analysis with monoclonal antibodies against PKR, and immune complexes were visualized by ECL.
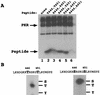
FIG. 9
In vitro phosphorylation and phosphoamino acid analysis of synthetic peptide substrates. (A) Transformants of strain H1817 (containing nonphosphorylatable eIF2α-S51A) bearing the wild-type PKR allele were grown in SGAL medium, and PKR was immunoprecipitated from whole-cell extracts as described in the legend to Fig. 6. Immune complexes were incubated in kinase reaction buffer in the presence of [γ-32P]ATP and 4 μg of a synthetic 20-mer peptide (lanes 2 to 6) or no added peptide (lane 1) for 15 min at 30°C. Radiolabeled samples were analyzed by Tricine-SDS-PAGE (10 to 20% polyacrylamide gradient gel). The gels were stained with Coomassie blue, dried under vacuum, and subjected to autoradiography. The wild-type peptide (whose sequence is shown on the left in panel B) was present in the reaction in lane 2. The mutant peptides analyzed in lanes 3 to 6 contained the indicated substitutions at positions 446 and 451. (B) Phosphoamino acid analysis of in vitro-labeled peptide substrates. In vitro kinase reactions were carried out as described above, and proteins were transferred to PVDF membranes and subjected to autoradiography. The strip of membrane containing the radiolabeled peptide was hydrolyzed with HCl, and soluble amino acids were resolved by thin-layer electrophoresis (see Materials and Methods), after which the phosphoamino acids were visualized by autoradiography. Unlabeled phosphoamino acid standards (phosphoserine [S], phosphothreonine [T], and phosphotyrosine [Y]) were separated along with each sample and visualized by ninhydrin staining. The positions of the standards are indicated to the right of each autoradiogram.
FIG. 10
Analysis of substitutions at Thr-882 and Thr-887 on GCN2 function in vivo and autokinase activity in vitro. (A) In vivo analysis of GCN2 function. Patches of transformants of strain H1894 containing the indicated GCN2 alleles on low-copy-number plasmids were grown to confluence in SD medium and replica plated to SD medium or SD medium supplemented with increasing concentrations of 3-AT ranging from 7.5 to 150 mM. Growth relative to that of the wild-type (WT) control was scored as follows: ++++, +++, ++, and +, the growth of the mutant was indistinguishable from that of the wild type on 3-AT concentrations of 150, 100, 75, and 10 mM, respectively; −, no growth at any of these 3-AT concentrations. The levels of in vitro kinase activities were ranked as follows: +, +/−, −/+, and −, activities that were identical to that of wild type, somewhat reduced, or greatly reduced relative to wild type, or undetectable. (B) Immunoblot analysis of GCN2 expression. Fifteen micrograms of whole-cell extracts prepared from transformants of strain H1894 bearing the indicated GCN2 alleles on low-copy-number plasmids were separated by SDS-PAGE with 6% polyacrylamide gels and transferred to PVDF membranes. Immunoblot analysis was performed with antibodies against GCN2 and GCD6 and ECL to detect the immune complexes. (C) In vitro autokinase activities of GCN2 proteins. Transformants of strain H1894 bearing the indicated GCN2 alleles on low-copy-number (lanes 1 to 10) or high-copy-number (lanes 11 to 14) plasmids were grown in liquid SD medium to an OD600 of 1.0, and GCN2 was immunoprecipitated from whole-cell extracts containing 150 μg of total protein with polyclonal antibodies against GCN2. Immune complexes were incubated in kinase reaction buffer in the presence of [γ-32P]ATP for 20 min at 30°C. Radiolabeled proteins were separated by SDS-PAGE with 4 to 16% gradient gels, transferred to PVDF membranes, and visualized by autoradiography (top panels). Immunodetection of GCN2 was performed on the same membranes with anti-GCN2 polyclonal antibodies and ECL to visualize the immune complexes (bottom panels). GCN2∼P, radiolabeled autophosphorylated GCN2.
FIG. 11
Analysis of phosphoamino acids produced by autophosphorylation of GCN2 in vitro. Transformants of strain H1894 bearing the indicated GCN2 alleles on high-copy-number plasmids were grown in liquid SD medium, and GCN2 was immunoprecipitated from whole-cell extracts in 5 or 10 reactions, each containing 200 to 400 μg of total yeast protein, with polyclonal antibodies against GCN2. Immune complexes were incubated in kinase reaction buffer in the presence of [γ-32P]ATP for 20 min at 30°C. Radiolabeled GCN2 proteins in the samples were separated by SDS-PAGE with 6% gels, transferred to PVDF membranes, and visualized by autoradiography. Strips of membranes containing GCN2 were treated with HCl to hydrolyze the protein, and soluble amino acids were resolved by thin-layer chromatography as described in Materials and Methods. Labeled phosphoamino acids were detected by autoradiography. The positions of unlabeled phosphoamino acid standards resolved in parallel and visualized by ninhydrin staining are indicated on the left as S (phosphoserine), T (phosphothreonine), and Y (phosphotyrosine).
Similar articles
- PKR and GCN2 kinases and guanine nucleotide exchange factor eukaryotic translation initiation factor 2B (eIF2B) recognize overlapping surfaces on eIF2alpha.
Dey M, Trieselmann B, Locke EG, Lu J, Cao C, Dar AC, Krishnamoorthy T, Dong J, Sicheri F, Dever TE. Dey M, et al. Mol Cell Biol. 2005 Apr;25(8):3063-75. doi: 10.1128/MCB.25.8.3063-3075.2005. Mol Cell Biol. 2005. PMID: 15798194 Free PMC article. - Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop.
Zhang F, Romano PR, Nagamura-Inoue T, Tian B, Dever TE, Mathews MB, Ozato K, Hinnebusch AG. Zhang F, et al. J Biol Chem. 2001 Jul 6;276(27):24946-58. doi: 10.1074/jbc.M102108200. Epub 2001 May 3. J Biol Chem. 2001. PMID: 11337501 - Activation of protein kinase PKR requires dimerization-induced cis-phosphorylation within the activation loop.
Dey M, Mann BR, Anshu A, Mannan MA. Dey M, et al. J Biol Chem. 2014 Feb 28;289(9):5747-57. doi: 10.1074/jbc.M113.527796. Epub 2013 Dec 13. J Biol Chem. 2014. PMID: 24338483 Free PMC article. - GCN20, a novel ATP binding cassette protein, and GCN1 reside in a complex that mediates activation of the eIF-2 alpha kinase GCN2 in amino acid-starved cells.
Vazquez de Aldana CR, Marton MJ, Hinnebusch AG. Vazquez de Aldana CR, et al. EMBO J. 1995 Jul 3;14(13):3184-99. doi: 10.1002/j.1460-2075.1995.tb07321.x. EMBO J. 1995. PMID: 7621831 Free PMC article. - Regulation of translation initiation by amino acids in eukaryotic cells.
Kimball SR. Kimball SR. Prog Mol Subcell Biol. 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6. Prog Mol Subcell Biol. 2001. PMID: 11575165 Review.
Cited by
- Reversal of pulmonary veno-occlusive disease phenotypes by inhibition of the integrated stress response.
Prabhakar A, Kumar R, Wadhwa M, Ghatpande P, Zhang J, Zhao Z, Lizama CO, Kharbikar BN, Gräf S, Treacy CM, Morrell NW, Graham BB, Lagna G, Hata A. Prabhakar A, et al. Nat Cardiovasc Res. 2024 Jul;3(7):799-818. doi: 10.1038/s44161-024-00495-z. Epub 2024 Jul 9. Nat Cardiovasc Res. 2024. PMID: 39196173 - Bacterial RNA induces myocyte cellular dysfunction through the activation of PKR.
Bleiblo F, Michael P, Brabant D, Ramana CV, Tai T, Saleh M, Parrillo JE, Kumar A, Kumar A. Bleiblo F, et al. J Thorac Dis. 2012 Apr 1;4(2):114-25. doi: 10.3978/j.issn.2072-1439.2012.01.07. J Thorac Dis. 2012. PMID: 22833816 Free PMC article. - A comprehensive map of the toll-like receptor signaling network.
Oda K, Kitano H. Oda K, et al. Mol Syst Biol. 2006;2:2006.0015. doi: 10.1038/msb4100057. Epub 2006 Apr 18. Mol Syst Biol. 2006. PMID: 16738560 Free PMC article. Review. - Type I Interferon at the Interface of Antiviral Immunity and Immune Regulation: The Curious Case of HIV-1.
Boasso A. Boasso A. Scientifica (Cairo). 2013;2013:580968. doi: 10.1155/2013/580968. Epub 2013 Dec 22. Scientifica (Cairo). 2013. PMID: 24455433 Free PMC article. Review.
References
- Adams J A, McGlone M L, Gibson R, Taylor S S. Phosphorylation modulates catalytic function and regulation in the cAMP-dependent protein kinase. Biochemistry. 1995;34:2447–2454. - PubMed
- Bischoff J R, Samuel C E. Mechanism of interferon action: the interferon-induced phosphoprotein P1 possesses a double-stranded RNA-dependent ATP-binding site. J Biol Chem. 1985;260:8237–8239. - PubMed
- Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases