Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300 - PubMed (original) (raw)
Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300
F Bex et al. Mol Cell Biol. 1998 Apr.
Abstract
The human T-cell leukemia virus type 1 Tax protein transforms human T lymphocytes, which can lead to the development of adult T-cell leukemia. Tax transformation is related to its ability to activate gene expression via the ATF/CREB and the NF-kappaB pathways. Transcriptional activation of these pathways is mediated by the actions of the related coactivators CREB binding protein (CBP) and p300. In this study, immunocytochemistry and confocal microscopy were used to localize CBP and p300 in cells expressing wild-type Tax or Tax mutants that are able to selectively activate gene expression from either the NF-kappaB or ATF/CREB pathway. Wild-type Tax colocalized with both CBP and p300 in nuclear bodies which also contained ATF-1 and the RelA subunit of NF-kappaB. However, a Tax mutant that selectively activates gene expression from only the ATF/CREB pathway colocalized with CBP but not p300, while a Tax mutant that selectively activates gene expression from only the NF-kappaB pathway colocalized with p300 but not CBP. In vitro and in vivo protein interaction studies indicated that the integrity of two independent domains of Tax delineated by these mutants was involved in the direct interaction of Tax with either CBP or p300. These studies are consistent with a model in which activation of either the NF-kappaB or the ATF/CREB pathway by specific Tax mutants is mediated by distinct interactions with related coactivator proteins.
Figures
FIG. 1
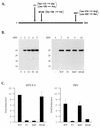
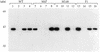
Differential activation of NF-κB and ATF/CREB pathways by Tax mutants. (A) A schematic of the 353-aa Tax protein. The positions of the amino acid changes for the three Tax mutants F1, M148, and M47 are shown. (B) Western blot analysis was performed on extracts prepared from BHK21 cells infected with SFV-Tax for 0 h (lane 1), 6 h (lane 2), 12 h (lane 3), 18 h (lane 4), or 24 h (lane 5). BHK21 cells were infected for 18 h with either control SFV (lane 6), SFV-Tax WT (lane 7), SFV-Tax F1 (lane 8), SFV-Tax M47 (lane 9), or SFV-Tax M148 (lane 10). Cellular extracts were subjected to electrophoresis on an SDS–15% polyacrylamide gel, and Western blot analysis was performed with a monoclonal antibody directed against Tax. Molecular weights (MW) in thousands are shown on the left. (C) BHK21 cells were transfected with either 1 μg of HTLV-1 CAT or HIV-1 CAT reporter plasmids; 5 h after transfection, the cells were infected with the control SFV (WT), SFV-Tax WT, SFV-Tax F1, SFV-Tax M47, or SFV-Tax M148, and they were harvested 24 h later. The CAT activity of the cell extracts was then determined, and the fold induction by either wild-type or mutant Tax was calculated for three independent experiments with the standard deviation indicated.
FIG. 2
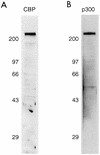
Specificity of the antibodies to CBP and p300. Western blot analysis was performed on BHK21 cell lysates with the rabbit polyclonal antibody to CBP (A) or the monoclonal antibody to p300 (B).
FIG. 3
CBP and p300 are distributed in distinct nuclear speckles. Uninfected BHK21 cells were fixed and analyzed by single immunofluorescence staining with either a rabbit polyclonal antibody that recognizes CBP (A) or a monoclonal antibody to p300 (C). The anti-CBP antibody was also used in combination with the monoclonal antibody to splicing factor Sm (B) or the monoclonal antibody to p300 (D). The anti-p300 antibody was used in combination with the monoclonal antibody to Sm (E). The DNA stain TO-PRO-3 was used to stain the nuclei (A and C). The panel on the right demonstrates the overlay of the blue and green staining or the red and green staining.
FIG. 4
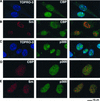
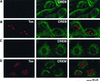
CBP and p300 colocalize with wild-type Tax in nuclear bodies. BHK21 cells were infected for 18 h with SFV-Tax (A and B), SFV-Tax M47 (C and D), or SFV-Tax M148 (E and F). The cells were fixed and stained by dual immunofluorescence with either a monoclonal antibody to Tax in combination with a rabbit polyclonal antibody that specifically recognizes CBP (A, C, and E) or a rabbit polyclonal antibody to Tax and a monoclonal antibody to p300 (B, D, and F). The panel on the right demonstrates the overlay of the red and green fluorescence staining.
FIG. 5
Independent domains in Tax mediate the interaction with CBP and p300. GST alone (lanes 2, 6, 10, and 14), GST-CBP (aa 450 to 682) (lanes 3, 7, 11, and 15), or GST-p300 (aa 436 to 662) (lanes 4, 8, 12, and 16) was bound to glutathione-agarose beads and incubated with 500 ng of purified wild-type (WT) Tax (lanes 2 to 4), Tax M47 (lanes 6 to 8), Tax M148 (lanes 10 to 12), or Tax F1 (lanes 14 to 16) protein. After extensive washing, the Tax proteins which remained attached to the beads were resolved by SDS-polyacrylamide gel electrophoresis and analyzed by Western blot analysis with a monoclonal antibody to Tax. Lanes 1, 5, 9, and 13 contain approximately 10% of the input Tax proteins. Molecular weights (in thousands) are shown on the left.
FIG. 6
In vivo association of Tax with CBP and p300. BHK21 cells were infected for 18 h with the control SFV (Mock) or with SFV expressing wild-type (WT) Tax or Tax mutants F1, M47, and M148. The cells were collected, and coimmunoprecipitation assays were performed with a monoclonal antibody to Tax. Western blot analysis was then performed with the cell lysates, using antibodies to Tax (A), CBP (B), and p300 (C), or on the immunoprecipitated complexes (IP), using antibodies to CBP (D) or p300 (E).
FIG. 7
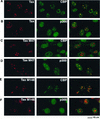
NF-κB RelA exhibits differential colocalization with the Tax mutants M47 and M148. BHK21 cells were infected for 18 h with control SFV (A), SFV-Tax (B), SFV-Tax F1 (C), SFV-Tax M47 (D), or SFV-Tax M148 (E). The cells were fixed and stained by dual immunofluorescence with a monoclonal antibody to Tax (left panel) and a rabbit polyclonal antibody to the RelA subunit of NF-κB (middle panel). The panel on the right demonstrates the overlay of the red and green fluorescence staining.
FIG. 8
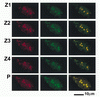
Assessment of Tax and RelA colocalization. Images from horizontal sections (Z series) were collected from a specimen of BHK21 cells infected with SFV-Tax. The cells were analyzed by dual immunofluorescence staining with antibodies to Tax and RelA. Z1 to Z4 are four of these sections, which were collected with 1-μm intervals. P is the projection of the Z series. The left panel displays the LRSC fluorescence of Tax, the middle panel displays the FITC fluorescence of RelA, and the right panel is the overlay of the red and green fluorescence staining.
FIG. 9
ATF-1 colocalizes with Tax in nuclear foci. BHK21 cells were infected for 18 h with control SFV (A), SFV-Tax (B), SFV-Tax F1 (C), SFV-Tax M47 (D), or SFV-Tax M148 (E). The cells were then fixed and stained by dual immunofluorescence with a rabbit polyclonal serum to Tax (left panel) and a monoclonal antibody that specifically recognizes ATF-1 (middle panel). The panel on the right demonstrates the overlay of the FITC and Texas Red fluorescence.
FIG. 10
Tax does not colocalize with the ATF/CREB proteins CREB and CREM. Either uninfected BHK21 cells (A and C) or BHK21 cells infected for 18 h with SFV-Tax (B and D) were fixed and analyzed by dual immunofluorescence staining with a Tax monoclonal antibody (left panel) and specific polyclonal antibodies that recognize either CREB (A and B) or CREM (C and D) (middle panel). The panel on the right demonstrates the overlay of the red and green fluorescence.
FIG. 11
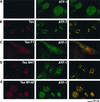
Tax colocalizes with CBP, p300, and ATF-1 in discrete nuclear foci present in HTLV-1-transformed lymphocytes. Either the HTLV-1-transformed MT2 lymphocytes (B, D, and F) or the T-lymphocyte Jurkat cells (A, C, and E) were centrifuged on microscope slides, fixed, and analyzed by dual immunofluorescence staining with a monoclonal antibody to Tax (left panel) and antibodies that specifically recognize either CBP (A and B) or p300 (C and D) (middle panel). The rabbit polyclonal antibody to Tax was used in conjunction with the monoclonal antibody to ATF-1 (E and F). The panel on the right demonstrates the overlay of the red and green fluorescence.
Similar articles
- Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4).
Gachon F, Thebault S, Peleraux A, Devaux C, Mesnard JM. Gachon F, et al. Mol Cell Biol. 2000 May;20(10):3470-81. doi: 10.1128/MCB.20.10.3470-3481.2000. Mol Cell Biol. 2000. PMID: 10779337 Free PMC article. - Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain.
Mulloy JC, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi MV, Cara A, Nicot C, Giam C, Franchini G. Mulloy JC, et al. J Virol. 1998 Nov;72(11):8852-60. doi: 10.1128/JVI.72.11.8852-8860.1998. J Virol. 1998. PMID: 9765430 Free PMC article. - Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template.
Lu H, Pise-Masison CA, Fletcher TM, Schiltz RL, Nagaich AK, Radonovich M, Hager G, Cole PA, Brady JN. Lu H, et al. Mol Cell Biol. 2002 Jul;22(13):4450-62. doi: 10.1128/MCB.22.13.4450-4462.2002. Mol Cell Biol. 2002. PMID: 12052856 Free PMC article. - Regulation of gene expression by HTLV-I Tax protein.
Bex F, Gaynor RB. Bex F, et al. Methods. 1998 Sep;16(1):83-94. doi: 10.1006/meth.1998.0646. Methods. 1998. PMID: 9774518 Review. - Regulation of NF-kappaB by the HTLV-1 Tax protein.
Li XH, Gaynor RB. Li XH, et al. Gene Expr. 1999;7(4-6):233-45. Gene Expr. 1999. PMID: 10440224 Free PMC article. Review.
Cited by
- Oncogenes and RNA splicing of human tumor viruses.
Ajiro M, Zheng ZM. Ajiro M, et al. Emerg Microbes Infect. 2014 Sep;3(9):e63. doi: 10.1038/emi.2014.62. Epub 2014 Sep 3. Emerg Microbes Infect. 2014. PMID: 26038756 Free PMC article. Review. - Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein.
Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. Lamsoul I, et al. Mol Cell Biol. 2005 Dec;25(23):10391-406. doi: 10.1128/MCB.25.23.10391-10406.2005. Mol Cell Biol. 2005. PMID: 16287853 Free PMC article. - Acetylation of the human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-kappaB pathway.
Lodewick J, Lamsoul I, Polania A, Lebrun S, Burny A, Ratner L, Bex F. Lodewick J, et al. Virology. 2009 Mar 30;386(1):68-78. doi: 10.1016/j.virol.2008.12.043. Epub 2009 Feb 5. Virology. 2009. PMID: 19200568 Free PMC article. - Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4).
Gachon F, Thebault S, Peleraux A, Devaux C, Mesnard JM. Gachon F, et al. Mol Cell Biol. 2000 May;20(10):3470-81. doi: 10.1128/MCB.20.10.3470-3481.2000. Mol Cell Biol. 2000. PMID: 10779337 Free PMC article. - Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone.
Robek MD, Ratner L. Robek MD, et al. J Virol. 1999 Jun;73(6):4856-65. doi: 10.1128/JVI.73.6.4856-4865.1999. J Virol. 1999. PMID: 10233947 Free PMC article.
References
- Arany Z, Sellers W R, Livingston D M, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. - PubMed
- Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
- Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. - PubMed
- Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-1 Tax induces cellular proteins that activate the κB element in the IL-2 receptor α gene. Science. 1988;241:1652–1655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous