HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism - PubMed (original) (raw)
HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism
R T Gandhi et al. J Exp Med. 1998.
Abstract
The mechanism by which HIV-1 induces CD4(+) T cell death is not known. A fundamental issue is whether HIV-1 primarily induces direct killing of infected cells or indirectly causes death of uninfected bystander cells. This question was studied using a reporter virus system in which infected cells are marked with the cell surface protein placental alkaline phosphatase (PLAP). Infection by HIV-PLAP of peripheral blood mononuclear cells (PBMCs) and T cell lines leads to rapid depletion of CD4(+) T cells and induction of apoptosis. The great majority of HIV-induced T cell death in vitro involves direct loss of infected cells rather than indirect effects on uninfected bystander cells. Because of its proposed role in HIV-induced cell death, we also examined the Fas (CD95/Apo1) pathway in killing of T cells by HIV-1. Infected PBMCs or CEM cells display no increase in surface Fas relative to uninfected cells. In addition, HIV-1 kills CEM and Jurkat T cells in the presence of a caspase inhibitor that completely blocks Fas-mediated apoptosis. HIV-1 also depletes CD4+ T cells in PBMCs from patients who have a genetically defective Fas pathway. These results suggest that HIV-1 induces direct apoptosis of infected cells and kills T cells by a Fas-independent mechanism.
Figures
Figure 1
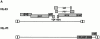
Genomic organization of HIV constructs. NL-PI is derived from NL43 and expresses all of the accessory genes of HIV-1. IRES indicates insertion of an internal ribosomal entry site (from encephalomyocarditis virus) which restores expression of nef in NL-PI. HXBnPLAP is derived from HXB-2D and does not express the accessory genes vpr, vpu and nef. Gray boxes indicate genes expressed by the virus; open boxes indicate genes that are not expressed.
Figure 1
Genomic organization of HIV constructs. NL-PI is derived from NL43 and expresses all of the accessory genes of HIV-1. IRES indicates insertion of an internal ribosomal entry site (from encephalomyocarditis virus) which restores expression of nef in NL-PI. HXBnPLAP is derived from HXB-2D and does not express the accessory genes vpr, vpu and nef. Gray boxes indicate genes expressed by the virus; open boxes indicate genes that are not expressed.
Figure 2
CD4+ T cell depletion after infection with HIV-1. (A) SupT1 and CEM cells were infected with either NL43 or NL-PI or mock-infected and the number of viable cells was determined by trypan blue exclusion on the indicated days after infection. (B) PBMCs from a normal donor were infected with different concentrations of NL43 (measured in nanograms per milliliter of p24) or mock-infected. Samples were stained daily for CD3, CD4, and CD8 and analyzed by flow cytometry. The percentage of CD4+ cells remaining on each day is plotted. Cells that were CD3+CD4−CD8− were considered to have downregulated CD4 and were included in the percentage of CD4+ T cells remaining. (C) CD4+ T cells were purified from a normal donor and infected with HXB2 or mock-infected. The number of viable cells was determined by trypan blue exclusion on the indicated days after infection. All experiments were performed at least three times and representative results are shown.
Figure 2
CD4+ T cell depletion after infection with HIV-1. (A) SupT1 and CEM cells were infected with either NL43 or NL-PI or mock-infected and the number of viable cells was determined by trypan blue exclusion on the indicated days after infection. (B) PBMCs from a normal donor were infected with different concentrations of NL43 (measured in nanograms per milliliter of p24) or mock-infected. Samples were stained daily for CD3, CD4, and CD8 and analyzed by flow cytometry. The percentage of CD4+ cells remaining on each day is plotted. Cells that were CD3+CD4−CD8− were considered to have downregulated CD4 and were included in the percentage of CD4+ T cells remaining. (C) CD4+ T cells were purified from a normal donor and infected with HXB2 or mock-infected. The number of viable cells was determined by trypan blue exclusion on the indicated days after infection. All experiments were performed at least three times and representative results are shown.
Figure 3
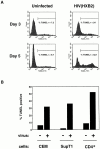
TUNEL assay on infected and uninfected CD4+ T cells. (A) CD4+ T cells from a normal donor were purified and infected with HXB2 or mock-infected. TUNEL assays were performed 3 and 5 d after infection. The TUNEL-positive gate was determined by analyzing each sample in parallel without TdT in the TUNEL reaction mix. (B) CEM and SupT1 cells were infected with NL43 or mock-infected and purified CD4+ T cells were infected with HXB2 or mock-infected. TUNEL assays were performed on days when the cell counts were declining. The percentage of TUNEL-positive cells is plotted for CEM, SupT1 (both day 6 after infection) and purified CD4+ T cells (day 5 after infection). These results are representative of three separate experiments.
Figure 4
Two-color flow cytometric analysis of cells for PLAP and TUNEL. CEM cells (A–C) and purified CD4+ T cells (D–F) were mock-infected or infected with NL-PI. CEM cells and purified CD4+ T cells were stained 4 and 5 d after infection, respectively, for PLAP and TUNEL. (A and D) uninfected cultures. (B and E) Infected cultures. (C and F) Infected cultures in which TdT was left out of the TUNEL reaction mix. The percentage of cells in each region is indicated in the corners of each plot. These results are representative of three separate experiments.
Figure 5
Two-color flow cytometric analysis of PBMC for Fas and PLAP. (A) Uninfected cells. (B) PBMCs on day 6 after infection with HXBnPLAP.
Figure 6
Sensitivity of CEM and SupT1 cells to anti-Fas antibody. Cells were incubated for 24 h in the presence of the indicated concentrations of mouse anti–human Fas IgM antibody (CH11). The percentage of apoptosis was assessed by staining the cells with propidium iodide and determining the fraction of cells that had hypodiploid DNA content.
Figure 7
The effect of the caspase inhibitor z-VAD-fmk on apoptosis induced by Fas and on killing of T cells by HIV-1. (A) Anti-Fas antibody (CH11) at 1 μg/ml was added to the indicated samples of CEM T cells that had been grown in the presence or absence of 50 μM of z-VAD-fmk. At 18 h after addition of anti-Fas antibody, the samples were analyzed for apoptosis by the TUNEL assay. (B) CEM T cells were infected with HXBnPLAP in the presence or absence of 50 μM z-VAD-fmk. Fresh z-VAD-fmk was added to the culture every 2 d. On the indicated days, equal sample volumes were counted for 1 min on a FACScan® and the number of cells with forward and side scatter characteristics consistent with viable cells was determined. This number in infected samples is plotted as a fraction of the number in the mock-infected culture.
Figure 8
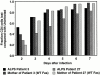
Fraction of CD4+ T cells lost after HIV-1 infection of PBMCs from patients with ALPS and normal controls. PBMCs from the patients characterized in Table 1 were infected with 200 ng/ml p24 of NL-PI and samples were stained daily for CD4 and PLAP. The fraction of CD4+ cells loss compared with uninfected is 1 − (percentage of CD4+ cells in the infected sample/percentage of CD4+ cells in the uninfected sample). Cells that were PLAP+ and CD4lo were considered to be infected cells that had downmodulated CD4, and, thus, were included in the determination of CD4+ cells present in the sample.
Similar articles
- CCR5 mediates Fas- and caspase-8 dependent apoptosis of both uninfected and HIV infected primary human CD4 T cells.
Algeciras-Schimnich A, Vlahakis SR, Villasis-Keever A, Gomez T, Heppelmann CJ, Bou G, Paya CV. Algeciras-Schimnich A, et al. AIDS. 2002 Jul 26;16(11):1467-78. doi: 10.1097/00002030-200207260-00003. AIDS. 2002. PMID: 12131184 - Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to a caspase-independent cell death.
Petit F, Arnoult D, Lelièvre JD, Moutouh-de Parseval L, Hance AJ, Schneider P, Corbeil J, Ameisen JC, Estaquier J. Petit F, et al. J Biol Chem. 2002 Jan 11;277(2):1477-87. doi: 10.1074/jbc.M102671200. Epub 2001 Oct 31. J Biol Chem. 2002. PMID: 11689551 - Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection.
Fraietta JA, Mueller YM, Yang G, Boesteanu AC, Gracias DT, Do DH, Hope JL, Kathuria N, McGettigan SE, Lewis MG, Giavedoni LD, Jacobson JM, Katsikis PD. Fraietta JA, et al. PLoS Pathog. 2013;9(10):e1003658. doi: 10.1371/journal.ppat.1003658. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130482 Free PMC article. Clinical Trial. - Role of FAS in HIV infection.
Dianzani U, Bensi T, Savarino A, Sametti S, Indelicato M, Mesturini R, Chiocchetti A. Dianzani U, et al. Curr HIV Res. 2003 Oct;1(4):405-17. doi: 10.2174/1570162033485131. Curr HIV Res. 2003. PMID: 15049427 Review. - Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS.
Alimonti JB, Ball TB, Fowke KR. Alimonti JB, et al. J Gen Virol. 2003 Jul;84(Pt 7):1649-1661. doi: 10.1099/vir.0.19110-0. J Gen Virol. 2003. PMID: 12810858 Review.
Cited by
- Role of T Cells in Microbial Pathogenesis.
Wang L, Song J. Wang L, et al. Pathogens. 2023 Nov 6;12(11):1321. doi: 10.3390/pathogens12111321. Pathogens. 2023. PMID: 38003786 Free PMC article. - Clearance of HIV-1 or SIV reservoirs by promotion of apoptosis and inhibition of autophagy: Targeting intracellular molecules in cure-directed strategies.
Chen M, Li M, Budai MM, Rice AP, Kimata JT, Mohan M, Wang J. Chen M, et al. J Leukoc Biol. 2022 Nov;112(5):1245-1259. doi: 10.1002/JLB.4MR0222-606. Epub 2022 Mar 31. J Leukoc Biol. 2022. PMID: 35362118 Free PMC article. Review. - Genetic signatures of HIV-1 envelope-mediated bystander apoptosis.
Joshi A, Lee RT, Mohl J, Sedano M, Khong WX, Ng OT, Maurer-Stroh S, Garg H. Joshi A, et al. J Biol Chem. 2014 Jan 31;289(5):2497-514. doi: 10.1074/jbc.M113.514018. Epub 2013 Nov 21. J Biol Chem. 2014. PMID: 24265318 Free PMC article. - Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection.
Hrimech M, Yao XJ, Bachand F, Rougeau N, Cohen EA. Hrimech M, et al. J Virol. 1999 May;73(5):4101-9. doi: 10.1128/JVI.73.5.4101-4109.1999. J Virol. 1999. PMID: 10196306 Free PMC article. - HIV-1 usurps transcription start site heterogeneity of host RNA polymerase II to maximize replication fitness.
Nikolaitchik OA, Islam S, Kitzrow JP, Duchon A, Cheng Z, Liu Y, Rawson JMO, Shao W, Nikolaitchik M, Kearney MF, Maldarelli F, Musier-Forsyth K, Pathak VK, Hu WS. Nikolaitchik OA, et al. Proc Natl Acad Sci U S A. 2023 Jun 6;120(23):e2305103120. doi: 10.1073/pnas.2305103120. Epub 2023 May 30. Proc Natl Acad Sci U S A. 2023. PMID: 37252967 Free PMC article.
References
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. - PubMed
- Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. - PubMed
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. - PubMed
- Mellors JW, Rinaldo C, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous