Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole - PubMed (original) (raw)
Review
Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole
N J Bryant et al. Microbiol Mol Biol Rev. 1998 Mar.
Abstract
Delivery of proteins to the vacuole of the yeast Saccharomyces cerevisiae provides an excellent model system in which to study vacuole and lysosome biogenesis and membrane traffic. This organelle receives proteins from a number of different routes, including proteins sorted away from the secretory pathway at the Golgi apparatus and endocytic traffic arising from the plasma membrane. Genetic analysis has revealed at least 60 genes involved in vacuolar protein sorting, numerous components of a novel cytoplasm-to-vacuole transport pathway, and a large number of proteins required for autophagy. Cell biological and biochemical studies have provided important molecular insights into the various protein delivery pathways to the yeast vacuole. This review describes the various pathways to the vacuole and illustrates how they are related to one another in the vacuolar network of S. cerevisiae.
Figures
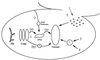
FIG. 1
Protein transport pathways to the yeast vacuole. Depicted are six pathways used by proteins to reach the vacuole in S. cerevisiae: 1, sorting of vacuolar proteins in the late Golgi (a) through a PVC and (b) via an alternative route; 2, endocytosis of proteins from the cell surface; 3, biosynthetic cytoplasm to vacuolar targeting; 4, autophagy (degradative cytoplasm to vacuolar targeting); 5, vacuolar inheritance from mother to daughter cells, using homotypic fusion to fuse vacuolar vesicles.
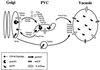
FIG. 2
Sorting of proteins from the late Golgi to the vacuole. Vacuolar proteins are sorted in vesicles bound for the vacuole at the level of the TGN. The soluble hydrolase, CPY, binds to its membrane receptor (Vps10p) in this compartment, and the receptor-ligand complex is delivered to the PVC. In the PVC, CPY dissociates from its receptor and travels on to the vacuole while Vps10p returns to the Golgi to bind more ligand. A second pathway from the TGN to the vacuole does not pass through this compartment and is taken by the vacuolar membrane proteins ALP and Vam3p. Interestingly, the two pathways converge at, or before, the vacuolar membrane.
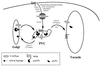
FIG. 3
Endocytosis of proteins from the plasma membrane to the vacuole. Proteins such as the mating pheromone receptor Ste2p are endocytosed to the vacuole from the plasma membrane, passing through at least two membrane-bound compartments en route. The requirements for this process are indicated here. The requirement of class E VPS genes for endocytosis pinpoints the PVC as a convergence point for the biosynthetic and endocytic pathways.
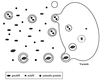
FIG. 4
Cytoplasm-to-vacuole targeting. API reaches the vacuole through the Cvt pathway. There is extensive overlap between the Cvt and autophagic pathways to the vacuole (see the text for details). Oligomerized pro-API is sequestered into cytoplasmic vesicles through a saturable, perhaps receptor-mediated process. These vesicles are then taken up by the vacuole via an autophagic mechanism. Breakdown of these vesicles requires PrB, resulting in the release of API into the vacuole lumen and its subsequent maturation.
FIG. 5
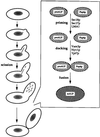
Vacuolar inheritance. Mother cells donate approximately 50% of their vacuolar material to growing buds. Protrusions from the vacuole grow toward the emerging bud, and a scission event leads to the formation of vesicles, which travel into the bud. A homotypic fusion event between these vesicles (consisting of a priming event, docking, and, finally, fusion) forms the vacuole of the daughter cell.
Similar articles
- Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment.
Vida TA, Huyer G, Emr SD. Vida TA, et al. J Cell Biol. 1993 Jun;121(6):1245-56. doi: 10.1083/jcb.121.6.1245. J Cell Biol. 1993. PMID: 8509446 Free PMC article. - A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole.
Rieder SE, Emr SD. Rieder SE, et al. Mol Biol Cell. 1997 Nov;8(11):2307-27. doi: 10.1091/mbc.8.11.2307. Mol Biol Cell. 1997. PMID: 9362071 Free PMC article. - Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route.
Bryant NJ, Piper RC, Gerrard SR, Stevens TH. Bryant NJ, et al. Eur J Cell Biol. 1998 May;76(1):43-52. doi: 10.1016/S0171-9335(98)80016-2. Eur J Cell Biol. 1998. PMID: 9650782 - The yeast lysosome-like vacuole: endpoint and crossroads.
Li SC, Kane PM. Li SC, et al. Biochim Biophys Acta. 2009 Apr;1793(4):650-63. doi: 10.1016/j.bbamcr.2008.08.003. Epub 2008 Aug 13. Biochim Biophys Acta. 2009. PMID: 18786576 Free PMC article. Review. - Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae.
Bowers K, Stevens TH. Bowers K, et al. Biochim Biophys Acta. 2005 Jul 10;1744(3):438-54. doi: 10.1016/j.bbamcr.2005.04.004. Biochim Biophys Acta. 2005. PMID: 15913810 Review.
Cited by
- H/KDEL receptors mediate host cell intoxication by a viral A/B toxin in yeast.
Becker B, Blum A, Gießelmann E, Dausend J, Rammo D, Müller NC, Tschacksch E, Steimer M, Spindler J, Becherer U, Rettig J, Breinig F, Schmitt MJ. Becker B, et al. Sci Rep. 2016 Aug 5;6:31105. doi: 10.1038/srep31105. Sci Rep. 2016. PMID: 27493088 Free PMC article. - Structural requirements for function of yeast GGAs in vacuolar protein sorting, alpha-factor maturation, and interactions with clathrin.
Mullins C, Bonifacino JS. Mullins C, et al. Mol Cell Biol. 2001 Dec;21(23):7981-94. doi: 10.1128/MCB.21.23.7981-7994.2001. Mol Cell Biol. 2001. PMID: 11689690 Free PMC article. - Regulation of gene expression by ambient pH in filamentous fungi and yeasts.
Peñalva MA, Arst HN Jr. Peñalva MA, et al. Microbiol Mol Biol Rev. 2002 Sep;66(3):426-46, table of contents. doi: 10.1128/MMBR.66.3.426-446.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208998 Free PMC article. Review. - The vacuole import and degradation pathway utilizes early steps of endocytosis and actin polymerization to deliver cargo proteins to the vacuole for degradation.
Brown CR, Dunton D, Chiang HL. Brown CR, et al. J Biol Chem. 2010 Jan 8;285(2):1516-28. doi: 10.1074/jbc.M109.028241. Epub 2009 Nov 5. J Biol Chem. 2010. PMID: 19892709 Free PMC article. - The TOR complex 1 is required for the interaction of multiple cargo proteins selected for the vacuole import and degradation pathway.
Alibhoy AA, Chiang HL. Alibhoy AA, et al. Commun Integr Biol. 2010 Nov;3(6):594-6. doi: 10.4161/cib.3.6.13241. Epub 2010 Nov 1. Commun Integr Biol. 2010. PMID: 21331250 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases