Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr - PubMed (original) (raw)
Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr
S Shah et al. J Cell Biol. 1998.
Abstract
A major question in nuclear import concerns the identity of the nucleoporin(s) that interact with the nuclear localization sequences (NLS) receptor and its cargo as they traverse the nuclear pore. Ligand blotting and solution binding studies of isolated proteins have attempted to gain clues to the identities of these nucleoporins, but the studies have from necessity probed binding events far from an in vivo context. Here we have asked what binding events occur in the more physiological context of a Xenopus egg extract, which contains nuclear pore subcomplexes in an assembly competent state. We have then assessed our conclusions in the context of assembled nuclear pores themselves. We have used immunoprecipitation to identify physiologically relevant complexes of nucleoporins and importin subunits. In parallel, we have demonstrated that it is possible to obtain immunofluorescence localization of nucleoporins to subregions of the nuclear pore and its associated structures. By immunoprecipitation, we find the nucleoporin Nup153 and the pore-associated filament protein Tpr, previously shown to reside at distinct sites on the intranuclear side of assembled pores, are each in stable subcomplexes with importin alpha and beta in Xenopus egg extracts. Importin subunits are not in stable complexes with nucleoporins Nup62, Nup93, Nup98, or Nup214/CAN, either in egg extracts or in extracts of assembled nuclear pores. In characterizing the Nup153 complex, we find that Nup153 can bind to a complete import complex containing importin alpha, beta, and an NLS substrate, consistent with an involvement of this nucleoporin in a terminal step of nuclear import. Importin beta binds directly to Nup153 and in vitro can do so at multiple sites in the Nup153 FXFG repeat region. Tpr, which has no FXFG repeats, binds to importin beta and to importin alpha/beta heterodimers, but only to those that do not carry an NLS substrate. That the complex of Tpr with importin beta is fundamentally different from that of Nup153 is additionally demonstrated by the finding that recombinant beta or beta45-462 fragment freely exchanges with the endogenous importin beta/Nup153 complex, but cannot displace endogenous importin beta from a Tpr complex. However, the GTP analogue GMP-PNP is able to disassemble both Nup153- and Tpr-importin beta complexes. Importantly, analysis of extracts of isolated nuclei indicates that Nup153- and Tpr-importin beta complexes exist in assembled nuclear pores. Thus, Nup153 and Tpr are major physiological binding sites for importin beta. Models for the roles of these interactions are discussed.
Figures
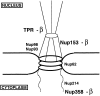
Figure 11
A model is shown summarizing the interactions of importin β with vertebrate nucleoporins under the in vivo–like conditions of the Xenopus egg extract and extracts of assembled nuclear pores. β indicates interaction with importin β in both the extract and in assembled pores. This occurred with nucleoporins Nup358, Nup153, and Tpr, although we were unable to assess Nup358 binding to β in rat liver nuclei due to a lack of specific anti-Nup358 antibody. A lack of β indicates no interaction of importin β with the designated nucleoporins was observed. This was found to be true for nucleoporins Nup214, Nup98, Nup93, and Nup62; Nup214 could only be assessed in Xenopus egg extracts.
Figure 1
Alignment of the Xenopus Nup153 partial cDNA with human Nup153. An alignment was performed using the human Nup153 aa sequence (aa 350–1475 are shown) and the aa sequence deduced from the partial Xenopus Nup153 cDNA. Identical aa are boxed while homologous aa are shaded in gray. The Xenopus Nup153 partial amino acid sequence is 38% identical to human Nup153, contains 38 FXFG-related repeats (16 FXFG), and 5 zinc finger repeats. The Zn fingers in Xenopus Nup153 are underlined. The Xenopus Nup153 sequence data is available from GenBank/EMBL/DDBJ under accession number AF045567.
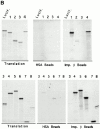
Figure 9
Mapping of the region of Nup153 that binds importin β in vitro_._ (A) Xenopus Nup153 contains a unique NH2-terminal domain (thin line), a zinc finger domain (box), and a COOH-terminal domain containing multiple FXFG repeats (thick line). Fragments were subcloned and expressed corresponding to aa 334–828 (Construct 1), aa 334–618 (Construct 2), aa 53–334 (Construct 3), and aa 618–1109 (Construct 4), aa 618–1219 (Construct 5), aa 828–1219 (Construct 6), aa 1110–1219 (Construct 7), and aa 618–828 (Construct 8) of the Xenopus Nup153 partial cDNA in Fig. 1. The cloned DNAs encoding the Nup153 fragments, shown in A, were transcribed and translated in [35S]methionine, along with a luciferase cDNA as a control. (B) An aliquot (2.5%) of the Nup153 radiolabeled protein fragments were analyzed by SDS-PAGE and exposed to film (Translation panel, Constructs 1–8); lane numbers are identical to the construct numbers. The individual radiolabeled Nup153 fragments were added to importin β–Sepharose beads or HSA–Sepharose beads and incubated for 2 h. The bound proteins were then separated using SDS-PAGE and the gel exposed to film. No binding of the Nup153 fragments to HSA-beads (HSA beads, top and bottom; Constructs 1–8) was observed. Constructs containing portions of the FXFG region of Nup153 bound strongly to importin β beads (Imp β beads, top and bottom; Constructs 1, 4, 5, 6, 7, and 8), while Constructs 2 and 3 did not (Imp β beads, Constructs 2 and 3).
Figure 9
Mapping of the region of Nup153 that binds importin β in vitro_._ (A) Xenopus Nup153 contains a unique NH2-terminal domain (thin line), a zinc finger domain (box), and a COOH-terminal domain containing multiple FXFG repeats (thick line). Fragments were subcloned and expressed corresponding to aa 334–828 (Construct 1), aa 334–618 (Construct 2), aa 53–334 (Construct 3), and aa 618–1109 (Construct 4), aa 618–1219 (Construct 5), aa 828–1219 (Construct 6), aa 1110–1219 (Construct 7), and aa 618–828 (Construct 8) of the Xenopus Nup153 partial cDNA in Fig. 1. The cloned DNAs encoding the Nup153 fragments, shown in A, were transcribed and translated in [35S]methionine, along with a luciferase cDNA as a control. (B) An aliquot (2.5%) of the Nup153 radiolabeled protein fragments were analyzed by SDS-PAGE and exposed to film (Translation panel, Constructs 1–8); lane numbers are identical to the construct numbers. The individual radiolabeled Nup153 fragments were added to importin β–Sepharose beads or HSA–Sepharose beads and incubated for 2 h. The bound proteins were then separated using SDS-PAGE and the gel exposed to film. No binding of the Nup153 fragments to HSA-beads (HSA beads, top and bottom; Constructs 1–8) was observed. Constructs containing portions of the FXFG region of Nup153 bound strongly to importin β beads (Imp β beads, top and bottom; Constructs 1, 4, 5, 6, 7, and 8), while Constructs 2 and 3 did not (Imp β beads, Constructs 2 and 3).
Figure 10
Nup153– and Tpr–importin β complexes are present in extracts of fully assembled nuclear pores. (A and B) Isolated rat liver nuclei were extracted with PBS Triton to release nuclear pore proteins. The nuclear extract was diluted and immunoprecipitated with anti-Nup153 (380), anti-Tpr, or rabbit IgG antibodies coupled to protein A–Sepharose, or with anti–rat Nup98 and anti-Nup93 antibodies uncoupled to Sepharose. The immunoprecipitates were split in half, electrophoresed on two separate gels, and immunoblotted. One blot (A) was probed with anti-Tpr (top strip; lanes 1–6), mAb 414 (middle strip), and anti–importin β antibodies (bottom strip). A second blot (B) was probed with a mix of anti–rat Nup98 and anti-Nup93 antibodies to demonstrate that Nup98 and Nup93 did immunoprecipitate (B, lanes 5 and 6). An aliquot of egg extract (0.05 μl) was run for size comparison (lane 1). Nup153 immunoprecipitated in a complex with importin β from rat liver nuclei (A, lane 3), as did Tpr (A, lane 4). Neither Nup98 nor Nup93 immunoprecipitated with importin β from rat liver nuclear extracts (A, lanes 5 and 6). (C) Identical immunoprecipitations were performed from rat liver nuclear extracts with anti-Tpr, anti-Nup153, or anti–rat Nup62 antisera. The blot was cut in two and probed with mAb 414 to detect Nup62 (top) and anti–importin β (bottom). Importin β coimmunoprecipitated with Nup153 (lane 2) and Tpr (lane 1), but not with Nup62 (lane 3).
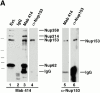
Figure 2
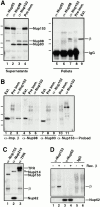
Antibody probes to Xenopus Nup153 and Tpr. (A) Immunoprecipitations were conducted with anti-Nup153 (380) antibody, mAb414, and rabbit IgG from Xenopus egg extract diluted 1:250 with buffer RL, as described in Materials and Methods. Pellets from the immunoprecipitations, along with an equivalent amount of diluted Xenopus egg extract (Ext.) for comparison, were then electrophoresed and immunoblotted independently with mAb 414 (lanes 1–4) and anti-Nup153 (380) antibody (lanes 5 and 6). The antibody used for immunoprecipitation is shown at the top of the figure, and the antibody used for immunoblotting is shown beneath the figures. Anti-Nup153 (380) immunoprecipitates a single major band of the expected size. (B) Soluble Xenopus egg extract (lane 1; 0.2 μl), and the membrane fraction of a Xenopus egg extract (lane 2; 0.2 μl of 10× membranes) were immunoblotted with anti-Tpr antibody. Anti-Tpr antibody recognizes a single band of the expected size in the soluble egg extract, which is not present in the membrane fraction.
Figure 2
Antibody probes to Xenopus Nup153 and Tpr. (A) Immunoprecipitations were conducted with anti-Nup153 (380) antibody, mAb414, and rabbit IgG from Xenopus egg extract diluted 1:250 with buffer RL, as described in Materials and Methods. Pellets from the immunoprecipitations, along with an equivalent amount of diluted Xenopus egg extract (Ext.) for comparison, were then electrophoresed and immunoblotted independently with mAb 414 (lanes 1–4) and anti-Nup153 (380) antibody (lanes 5 and 6). The antibody used for immunoprecipitation is shown at the top of the figure, and the antibody used for immunoblotting is shown beneath the figures. Anti-Nup153 (380) immunoprecipitates a single major band of the expected size. (B) Soluble Xenopus egg extract (lane 1; 0.2 μl), and the membrane fraction of a Xenopus egg extract (lane 2; 0.2 μl of 10× membranes) were immunoblotted with anti-Tpr antibody. Anti-Tpr antibody recognizes a single band of the expected size in the soluble egg extract, which is not present in the membrane fraction.
Figure 3
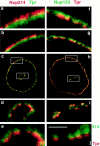
Distinct immunofluorescent localization of Nup153, Nup214, and Tpr. Anti-Nup153 (380) antibody was covalently linked to fluorescein, whereas anti-Tpr antibody was directly linked to rhodamine or fluorescein independently, using isothiocyanate derivatives (RITC and FITC, respectively) as described in Materials and Methods. For immunostaining and colocalization, anti-Nup214 was first used to immunostain formaldehyde fixed XL177 cells. After incubation of the cells with goat anti–rabbit-RITC antibody and subsequent blocking of this antibody with excess IgG, cells were then stained with FITC–anti-Tpr antibody, as described in Materials and Methods (a–e). The anti-Tpr stain is punctate and lies more to the nuclear interior than the anti–Nup214 stain. To compare localization of Nup153 and Tpr (f–i), XL177 cells were costained with FITC–anti-Nup153 (380) antibody and RITC–anti-Tpr antibody. Nup153 and Tpr show a more overlapping localization, although a large amount of Tpr staining still lies more intranuclearly than that of Nup153. A low magnification of the colocalization is shown in c and h, a 4× higher magnification view in panels immediately above and below (b, d, g, i), and an 8× higher magnified view lies above and below these (a, e, f). The nuclei shown contain internal tunnels of nuclear envelope, with magnified views of the tunnel shown in d, e, and i. Magnified views of the circumferential nuclear envelope are shown in a, b, f, and g. Note that the Nup214 staining (a–e) is always closer to the cytoplasm, whereas the Tpr staining (a–i) is always more proximal to the nuclear interior. j shows mAb414 (Oregon Green) and anti-Tpr (RITC) staining of a XL177 tissue culture cell, showing a segment of the nuclear envelope. Bar, ∼1 μm.
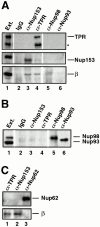
Figure 4
Importin β is in a complex with Nup153, but not Nup62, Nup93, Nup98, or Nup214. (A) Immunoprecipitations were conducted from Xenopus egg extract diluted in buffer RL using anti-Nup153 (361), protein A–purified 361 preimmune, anti-Nup93, and anti-Xenopus Nup98 antibodies. The antibody used for immunoprecipitation is shown at the top of each lane. The supernatants of the immunoprecipitates (lanes 1–4) were immunoblotted with a mixture of anti-Nup93, anti-Xenopus Nup98, and anti-rat Nup153 antibodies to demonstrate that each nucleoporin had been quantitatively immunoprecipitated. Lane 1 is depleted of Nup93, lane 2 is depleted of Nup98, and lane 3 is depleted of the great majority of Nup153. The proteins of the pellets immunoprecipitated from 2.5 μl Xenopus egg extract (lanes 5–8) were immunoblotted with anti–importin β antiserum. Soluble Xenopus egg extract (0.05 μl) is shown (lane 9) to indicate where importin β migrates. Importin β coimmunoprecipitates with Nup153 (lane 7), but not with Nup93 (lane 5) or Nup98 (lane 6). (B) Immunoprecipitation from soluble Xenopus egg extract diluted in buffer RL were performed using anti-Nup153 (380) and preimmune antisera bound to protein A–Sepharose. Immunoprecipitated protein was eluted with glycine and separated on an 8% SDS-PAGE gel along with aliquots of Xenopus egg extract (lanes 1, 4, and 7). Proteins were transferred to PVDF and analyzed by immunoblotting with the following antibodies: anti–importin β (lanes 1–3), anti-Xenopus Nup98 (lanes 4–6), anti-Nup93 (lanes 7–9), and anti-Nup153 (lanes 10 and 11). Note that importin β is detected in Nup153 immunoprecipitates and Xenopus egg extract (lane 1 and 3), but not in a preimmune immunoprecipitate (lane 2). Also note that Nup98 and Nup93 are detected in Xenopus egg extract (lanes 4 and 7), but not in preimmune or Nup153 immunoprecipitates (lanes 5, 6, 8, and 9). (C) Immunoprecipitates of Nup153, Nup214, and Tpr were separated, transferred, and probed with anti–importin β, anti-Tpr, and mAb414 to detect Nup214, Nup153, and Nup62. An asterisk marks a breakdown product of Tpr. Importin β immunoprecipitates with Nup153 (lane 1) and Tpr (lane 3), but not with Nup214 (lane 2). (D) Immunoprecipitates of Nup153, Nup62, and control IgG were separated, transferred, and probed with anti–importin β, and mAb414 to detect Nup62. Full-length recombinant importin β immunoprecipitates with Nup153 (lane 2), but not with Nup62 (lane 4) or nonspecific IgG (lane 6).
Figure 5
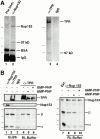
Stable independent complexes of Nup153 and Tpr with importin β exist. (A) Coimmunoprecipitation of a ∼97-kD band with Nup153 and with Tpr. Anti-Nup153 (380) and preimmune antibodies were bound to protein A–Sepharose (lane 1 and 2, respectively) and used for immunoprecipitation from 20 μl soluble Xenopus egg extract, as described in Materials and Methods. The immunoprecipitates were boiled with sample buffer, separated using SDS-PAGE (8%), and silver stained. In addition to Nup153, a prominent protein band of ∼97 kD immunoprecipitated with anti-Nup153 (lane 2). The markers for this gel are 193, 112, 86, and 70 kD. A separate experiment was performed with anti-Tpr antiserum; a band of ∼97 kD was observed to precipitate with Tpr (lane 3). The markers for this gel are 205, 116, and 97.4 kD. (B) Immunoprecipitations from soluble Xenopus egg extract were performed using anti-153 (380), anti-Tpr, or rabbit IgG antibodies coupled to protein A–Sepharose. Before antibody addition, the extract was diluted (1:100) in either ELBS buffer (lanes 1–3), or RL buffer (lanes 4 and 8). In other incubations Xenopus egg extract was preincubated with GMP-PNP (lanes 6 and 10) or AMP-PNP (lanes 5 and 9) before dilution with buffer RL and immunoprecipitation, as above. The immunoprecipitated proteins and an aliquot of soluble Xenopus egg extract (lane 7) were analyzed by gel electrophoresis and transfer to PVDF. The blot on the left was cut and probed by immunoblotting with anti-Tpr (top), anti-Nup153 (381; middle) or a mix of anti–importin α and β antibodies (bottom). The blot on the right was probed with a mix of anti-Nup153, importin β, and importin α antibodies. Importin α and β immunoprecipitate with anti-Nup153 (lane 1) and anti-Tpr (lane 2) antisera. The binding of importin β to Nup153 and Tpr is stable in both ELBS (lanes 1 and 2) and RL (lanes 4, 5, 8, and 9) buffers; however, the binding to importin α is disrupted in the more stringent RL buffer (lanes 4, 5, 8, and 9). GMP-PNP disrupts the complex of importin β with Tpr (lane 6) and Nup153 (lane 10). The differences in the amounts of TPR in Fig. 5, lanes 4–6 are due to inefficient transfer of the very large Tpr protein (∼270 kD) from the 8% gel used to simultaneously view importin α; reelectrophoresis of the samples revealed only small differences in the amounts of Tpr per lane (data not shown).
Figure 6
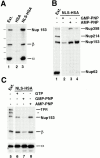
Nup153, but not Tpr, binds to importin α/β heterodimer in the NLS-bound form. (A) Anti-HSA antibody coupled to protein A–Sepharose was added to Xenopus egg extract (diluted 1:100 in buffer ELBS) containing 2.4 μg HSA (lane 2) or 1.9 μg NLS-HSA (lane 3). The proteins that bound to the NLS-HSA/anti-HSA/ Protein A beads were eluted and immunoblotted with a mix of anti–importin α and β and anti-Nup153 (381) antibodies. The proteins present in 0.2 μl of soluble Xenopus egg extract are shown in lane 1 for comparison. Importin α, importin β, and Nup153 all bind to an NLS-HSA affinity column (lane 3), but not to an HSA column (lane 2). (B) GMP-PNP disrupts Nup153 bound to importin α/β– NLS complexes. NLS-HSA/anti-HSA Sepharose beads were added to Xenopus egg extract pretreated with GMP-PNP, AMP-PNP, or no addition (lanes 2–4, respectively). Before bead addition, extracts were diluted with buffer ELBS and supplemented with 1.9 μg of NLS-HSA per aliquot. The NLS-HSA bead mixtures were incubated in extract for 2 h. Then bound proteins were eluted, electrophoresed, and immunoblotted with mAb414 (lanes 1–4). Soluble Xenopus egg extract (0.2 μl) was immunoblotted in parallel (lane 1) to indicate the nucleoporins recognized in the total extract by mAb 414 (Nup358, Nup214, Nup153, and Nup62). Much of the Nup358 and Nup153, but only a trace of Nup62, bind to the NLS-HSA affinity column (lanes 3 and 4). GMP-PNP disrupts the majority of this binding (lane 2), whereas AMP-PNP does not (lane 3). (C) Tpr does not bind to importin α/β–NLS complexes. The experiment in (A) was repeated, and the bound proteins were immunoblotted with a mix of anti-Tpr, anti-Nup153 (381), anti–importin α and β (lanes 5–8). Nup153 and importin α and β bind to the NLS-HSA beads (lane 6), but Tpr does not (lane 6). GMP-PNP releases the importin β and Nup153 from the beads (lane 7), whereas AMP-PNP does not (lane 8). (An asterisk denotes a breakdown product of Tpr.)
Figure 8
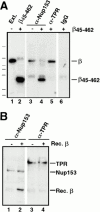
β45-462 removes nucleoporins Nup153 and Nup358 bound to an NLS-HSA column. Xenopus extract was diluted and supplemented with NLS-HSA (1.9 μg/immunoprecipitation). Importin β45–462 was added to half (lanes 2 and 4), while the equivalent amount of buffer was added to the other half (lanes 1 and 3). Anti-HSA antibody coupled to protein A–Sepharose was then added. Bound proteins were eluted from the NLS-HSA/anti-HSA beads with glycine. A portion of each was electrophoresed and immunoblotted with a mix of anti–importin α and β antisera (lanes 1 and 2), or a mix of mAb 414, anti-Nup214, anti-Xenopus Nup98, and anti-Nup93 (lanes 3–5). An aliquot of Xenopus egg cytosol (0.2 μl; lane 5) indicates that Nup358, Nup214, Nup153, Nup98, Nup93, and Nup62 would have been detected if bound to the NLS-HSA beads. Of these, only Nup358 and Nup153 bound (lane 3); both were displaced by added β45–462 (lane 4). This indicates that Nup153 and Nup358 bind to the NLS-HSA column through importin β.
Figure 7
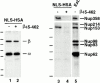
The effects of β45–462 on Nup153– and Tpr–importin β complexes. (A) Immunoprecipitations using anti-Nup153 (380), anti-Tpr, or rabbit IgG antibodies coupled to protein A–Sepharose were performed on soluble Xenopus egg extract diluted in buffer RL to which had been added the importin β fragment β45–462 buffer RL. The immunoprecipitates were eluted with glycine and immunoblotted with anti–importin β antiserum. Lane 1 shows an aliquot of egg extract and lane 2, an aliquot of recombinant importin fragment, β45–462; Importin β immunoprecipitates with Nup153 (lane 3) and Tpr (lane 5). Recombinant importin fragment β45–462 displaces full-length importin β on Nup153 (lane 4), but is unable to displace importin β on Tpr (lane 5). No binding of either form of importin β is observed on control rabbit IgG (lane 6). (B) Recombinant importin β is able to bind directly to Nup153, but not to Tpr. Anti-Nup153 (380) and anti-Tpr immunoprecipitates lacking full-length importin β were isolated from Xenopus egg extracts by pretreating the extract with GMP-PNP before immunoprecipitation and washing the beads with high salt. The washed immunoprecipitate beads from above were split into 2 tubes and either recombinant importin β in buffer RL (lanes 2 and 4), or buffer RL alone (lanes 1 and 3) was added. After incubation for 90 min, the beads were washed and bound proteins were electrophoresed, and immunoblotted with a mix of anti-Nup153 (381) and anti–importin β antibodies (lanes 1 and 2), or a mix of anti-Tpr and anti–importin β antibodies (lanes 3 and 4). Recombinant importin β was able to bind directly to Nup153 (lane 2), but not to Tpr (lane 4).
Similar articles
- Separate nuclear import pathways converge on the nucleoporin Nup153 and can be dissected with dominant-negative inhibitors.
Shah S, Forbes DJ. Shah S, et al. Curr Biol. 1998 Dec 17-31;8(25):1376-86. doi: 10.1016/s0960-9822(98)00018-9. Curr Biol. 1998. PMID: 9889100 - Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export.
Vasu S, Shah S, Orjalo A, Park M, Fischer WH, Forbes DJ. Vasu S, et al. J Cell Biol. 2001 Oct 29;155(3):339-54. doi: 10.1083/jcb.200108007. Epub 2001 Oct 29. J Cell Biol. 2001. PMID: 11684705 Free PMC article. - The nucleoporin nup153 plays a critical role in multiple types of nuclear export.
Ullman KS, Shah S, Powers MA, Forbes DJ. Ullman KS, et al. Mol Biol Cell. 1999 Mar;10(3):649-64. doi: 10.1091/mbc.10.3.649. Mol Biol Cell. 1999. PMID: 10069809 Free PMC article. - Molecular mechanisms of nuclear protein transport.
Moroianu J. Moroianu J. Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review. - Molecular mechanism of translocation through nuclear pore complexes during nuclear protein import.
Stewart M, Baker RP, Bayliss R, Clayton L, Grant RP, Littlewood T, Matsuura Y. Stewart M, et al. FEBS Lett. 2001 Jun 8;498(2-3):145-9. doi: 10.1016/s0014-5793(01)02489-9. FEBS Lett. 2001. PMID: 11412846 Review.
Cited by
- Picornaviruses and nuclear functions: targeting a cellular compartment distinct from the replication site of a positive-strand RNA virus.
Flather D, Semler BL. Flather D, et al. Front Microbiol. 2015 Jun 18;6:594. doi: 10.3389/fmicb.2015.00594. eCollection 2015. Front Microbiol. 2015. PMID: 26150805 Free PMC article. Review. - Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells.
Sheth U, Pitt J, Dennis S, Priess JR. Sheth U, et al. Development. 2010 Apr;137(8):1305-14. doi: 10.1242/dev.044255. Epub 2010 Mar 10. Development. 2010. PMID: 20223759 Free PMC article. - Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export.
Frosst P, Guan T, Subauste C, Hahn K, Gerace L. Frosst P, et al. J Cell Biol. 2002 Feb 18;156(4):617-30. doi: 10.1083/jcb.200106046. Epub 2002 Feb 11. J Cell Biol. 2002. PMID: 11839768 Free PMC article. - Nuclear import of the ran exchange factor, RCC1, is mediated by at least two distinct mechanisms.
Nemergut ME, Macara IG. Nemergut ME, et al. J Cell Biol. 2000 May 15;149(4):835-50. doi: 10.1083/jcb.149.4.835. J Cell Biol. 2000. PMID: 10811825 Free PMC article. - Nup98 is a mobile nucleoporin with transcription-dependent dynamics.
Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Griffis ER, et al. Mol Biol Cell. 2002 Apr;13(4):1282-97. doi: 10.1091/mbc.01-11-0538. Mol Biol Cell. 2002. PMID: 11950939 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous