Brefeldin A-inhibited guanine nucleotide-exchange activity of Sec7 domain from yeast Sec7 with yeast and mammalian ADP ribosylation factors - PubMed (original) (raw)
Brefeldin A-inhibited guanine nucleotide-exchange activity of Sec7 domain from yeast Sec7 with yeast and mammalian ADP ribosylation factors
M Sata et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The Saccharomyces cerevisiae Sec7 protein (ySec7p), which is an important component of the yeast secretory pathway, contains a sequence of approximately 200 amino acids referred to as a Sec7 domain. Similar Sec7 domain sequences have been recognized in several guanine nucleotide-exchange proteins (GEPs) for ADP ribosylation factors (ARFs). ARFs are approximately 20-kDa GTPases that regulate intracellular vesicular membrane trafficking and activate phospholipase D. GEPs activate ARFs by catalyzing the replacement of bound GDP with GTP. We, therefore, undertook to determine whether a Sec7 domain itself could catalyze nucleotide exchange on ARF and found that it exhibited brefeldin A (BFA)-inhibitable ARF GEP activity. BFA is known to inhibit ARF GEP activity in Golgi membranes, thereby causing reversible apparent dissolution of the Golgi complex in many cells. The His6-tagged Sec7 domain from ySec7p (rySec7d) synthesized in Escherichia coli enhanced binding of guanosine 5'-[gamma-[35S]thio]triphosphate by recombinant yeast ARF1 (ryARF1) and ryARF2 but not by ryARF3. The effects of rySec7d on ryARF2 were inhibited by BFA in a concentration-dependent manner but not by inactive analogues of BFA (B-17, B-27, and B-36). rySec7d also promoted BFA-sensitive guanosine 5'-[gamma-thio]triphosphate binding by nonmyristoylated recombinant human ARF1 (rhARF1), rhARF5, and rhARF6, although the effect on rhARF6 was very small. These results are consistent with the conclusion that the yeast Sec7 domain itself contains the elements necessary for ARF GEP activity and its inhibition by BFA.
Figures
Figure 1
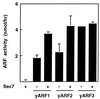
Effect of rySec7d on activation of CTA by ryARF1, ryARF2, and ryARF3. First, 0.5 μg of ryARF1, ryARF2, or ryARF3 and 10 μM GTP[γS] were incubated without or with 0.5 μg of rySec7d at 37°C for 40 min in 20 mM Tris⋅HCl (pH 8.0) containing 1 mM EDTA, 1 mM NaN3, 10 mM DTT, 0.25 M sucrose, 5 mM MgCl2, 15 μg of BSA, and 20 μg of phosphatidylserine. Components needed to quantify ARF stimulation of cholera toxin-catalyzed ADP ribosylagmatine formation were then added, and incubation was continued for 60 min at 30°C before separation of the product for radioassay. ARF activity is the difference between CTA activity with ARF and that without ARF. The experiment was repeated twice.
Figure 2
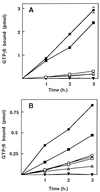
Effect of temperature on rySec7d stimulation of GTP[γS] binding to ryARF2. (A) ryARF2 (1 μg, 50 pmol) and 4 μM [35S]GTP[γS] were incubated at 30°C without (▪ and □) or with (• and ○) 0.2 μg (8.3 pmol) of rySec7d for the indicated time before collection of proteins for radioassay. Some samples (□ and ○) also contained 6 μg of BFA (0.2 mM). (B) ryARF2 (50 pmol) and 4 μM [35S]GTP[γS] were incubated at 24°C without (•) or with 0.2 μg (□) or 0.4 μg (▪) of rySec7d for the indicated time. To some samples, after incubation for 15 sec with 0.2 μg of rySec7d, a second addition of 0.2 μg was made (▴). rySec7d, 0.2 μg (▿) or 0.4 μg (▾) was also incubated without ARF. (C) ryARF2 (50 pmol) and 4 μM [35S]GTP[γS] were incubated at 4°C without (○) or with (•) 0.2 μg of rySec7d for the indicated time. rySec7d (0.2 μg) was also incubated without yeast ARF2 (▪).
Figure 3
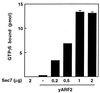
Effect of rySec7d concentration on binding of GTP[γS] to ryARF2. ryARF2 (50 pmol) and 4 μM [35S]GTP[γS] were incubated with the indicated amount of rySec7d at 4°C for 3 h before radioassay of protein-bound [35S]GTP[γS]. rySec7d (2 μg) incubated alone bound no GTP[γS].
Figure 4
Effect of rySec7d on GTP[γS] binding to ryARFs and rhARFs. (A) Fifty picomoles of ryARF1 (□ and ▪), ryARF2 (○ and •), or ryARF3 (▵ and ▴) and 4 μM [35S]GTP[γS] were incubated without (□, ○, and ▵) or with 0.2 μg (▪, •, and ▴) of rySec7d for the indicated time before radioassay of protein-bound [35S]GTP[γS]. rySec7d (0.2 μg, ▾) did not bind GTP[γS]. (B) One hundred picomoles of rhARF1 (□ and ▪), rhARF5 (○ and •), or rhARF6 (▵ and ▴) were incubated without (open symbols) or with (solid symbols) 2 μg of rySec7d and 4 μM [35S]GTP[γS] before radioassay of protein-bound [35S]GTP[γS]. rySec7d (2 μg; ▾) was incubated as control.
Figure 5
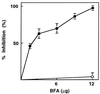
Inhibition of rySec7d-stimulated GTP[γS] binding to ryARF2 by BFA. Fifty picomoles of ryARF2 and 4 μM [35S]GTP[γS] were incubated with 0.2 μg of rySec7d and the indicated amounts of BFA (•) or MeOH (○) for 3 h at 4°C before radioassay of protein-bound [35S]GTP[γS]. The decrease in binding caused by BFA is expressed as percentage inhibition of binding in its absence. All assays contained 1.2% CH3OH, the amount added with 12 μg of BFA.
Similar articles
- Cytohesin-1, a cytosolic guanine nucleotide-exchange protein for ADP-ribosylation factor.
Meacci E, Tsai SC, Adamik R, Moss J, Vaughan M. Meacci E, et al. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1745-8. doi: 10.1073/pnas.94.5.1745. Proc Natl Acad Sci U S A. 1997. PMID: 9050849 Free PMC article. - Brefeldin A inhibited activity of the sec7 domain of p200, a mammalian guanine nucleotide-exchange protein for ADP-ribosylation factors.
Morinaga N, Adamik R, Moss J, Vaughan M. Morinaga N, et al. J Biol Chem. 1999 Jun 18;274(25):17417-23. doi: 10.1074/jbc.274.25.17417. J Biol Chem. 1999. PMID: 10364170 - Guanine nucleotide exchange on ADP-ribosylation factors catalyzed by cytohesin-1 and its Sec7 domain.
Pacheco-Rodriguez G, Meacci E, Vitale N, Moss J, Vaughan M. Pacheco-Rodriguez G, et al. J Biol Chem. 1998 Oct 9;273(41):26543-8. doi: 10.1074/jbc.273.41.26543. J Biol Chem. 1998. PMID: 9756891 - Activation of toxin ADP-ribosyltransferases by eukaryotic ADP-ribosylation factors.
Moss J, Vaughan M. Moss J, et al. Mol Cell Biochem. 1999 Mar;193(1-2):153-7. Mol Cell Biochem. 1999. PMID: 10331652 Review. - Activation of toxin ADP-ribosyltransferases by the family of ADP-ribosylation factors.
Vaughan M, Moss J. Vaughan M, et al. Adv Exp Med Biol. 1997;419:315-20. doi: 10.1007/978-1-4419-8632-0_41. Adv Exp Med Biol. 1997. PMID: 9193671 Review.
Cited by
- Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex.
Yamaji R, Adamik R, Takeda K, Togawa A, Pacheco-Rodriguez G, Ferrans VJ, Moss J, Vaughan M. Yamaji R, et al. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2567-72. doi: 10.1073/pnas.97.6.2567. Proc Natl Acad Sci U S A. 2000. PMID: 10716990 Free PMC article. - AtTRAPPC11/ROG2: A Role for TRAPPs in Maintenance of the Plant _Trans_-Golgi Network/Early Endosome Organization and Function.
Rosquete MR, Worden N, Ren G, Sinclair RM, Pfleger S, Salemi M, Phinney BS, Domozych D, Wilkop T, Drakakaki G. Rosquete MR, et al. Plant Cell. 2019 Aug;31(8):1879-1898. doi: 10.1105/tpc.19.00110. Epub 2019 Jun 7. Plant Cell. 2019. PMID: 31175171 Free PMC article. - Disordered hinge regions of the AP-3 adaptor complex promote vesicle budding from the late Golgi in yeast.
Leih M, Plemel RL, West M, Angers CG, Merz AJ, Odorizzi G. Leih M, et al. J Cell Sci. 2024 Nov 1;137(21):jcs262234. doi: 10.1242/jcs.262234. Epub 2024 Nov 8. J Cell Sci. 2024. PMID: 39330471 Free PMC article. - Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers.
Cox R, Mason-Gamer RJ, Jackson CL, Segev N. Cox R, et al. Mol Biol Cell. 2004 Apr;15(4):1487-505. doi: 10.1091/mbc.e03-06-0443. Epub 2004 Jan 23. Mol Biol Cell. 2004. PMID: 14742722 Free PMC article. - The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes.
Drake MT, Zhu Y, Kornfeld S. Drake MT, et al. Mol Biol Cell. 2000 Nov;11(11):3723-36. doi: 10.1091/mbc.11.11.3723. Mol Biol Cell. 2000. PMID: 11071902 Free PMC article.
References
- Achstetter T, Franzusoff A, Field C, Schekman R. J Biol Chem. 1988;263:11711–11717. - PubMed
- Franzusoff A, Lauzé E, Howell K E. Nature (London) 1992;355:173–175. - PubMed
- Shevell D E, Leu W M, Gillmor C S, Xia G, Feldmann K A, Chua N H. Cell. 1994;77:1051–1062. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases