Traumatic spinal cord injury induces nuclear factor-kappaB activation - PubMed (original) (raw)
Traumatic spinal cord injury induces nuclear factor-kappaB activation
J R Bethea et al. J Neurosci. 1998.
Abstract
Inflammatory responses are a major component of secondary injury and play a central role in mediating the pathogenesis of acute and chronic spinal cord injury (SCI). The nuclear factor-kappaB (NF-kappaB) family of transcription factors is required for the transcriptional activation of a variety of genes regulating inflammatory, proliferative, and cell death responses of cells. In this study we examined the temporal and cellular expression of activated NF-kappaB after traumatic SCI. We used a contusion model (N.Y.U. Impactor) to initiate the early biochemical and molecular changes that occur after traumatic injury to reproduce the pathological events associated with acute inflammation after SCI. The activation and cellular distribution of activated NF-kappaB was evaluated by using a monoclonal antibody that selectively recognizes activated p65 in a NF-kappaB dimer. Immunohistochemical and Western blot analyses demonstrated that NF-kappaB activation occurred as early as 0.5 hr postinjury and persisted for at least 72 hr. Using electrophoretic mobility shift assays (EMSA), we demonstrate that NF-kappaB is activated after SCI. In our immunohistochemical, Western, and EMSA experiments there are detectable levels of activated NF-kappaB in our control animals. Using double-staining protocols, we detected activated NF-kappaB in macrophages/microglia, endothelial cells, and neurons within the injured spinal cord. Colocalization of activated NF-kappaB with the NF-kappaB-dependent gene product, inducible nitric oxide synthase (iNOS), suggests functional implications for this transcription factor in the pathogenesis of acute spinal cord injury. Although there is considerable evidence for the involvement of an inflammatory reaction after traumatic SCI, this is the first evidence for the activation of NF-kappaB after trauma. Strategies directed at blocking the initiation of this cascade may prove beneficial as a therapeutic approach for the treatment of acute SCI.
Figures
Fig. 1.
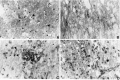
Histopathological analysis of spinal cords at 24 and 72 hr after contusion injury. Photomicrographs show necrosis, infiltration of leukocytes, and white matter vacuolization.A, At 24 hr after SCI, necrotic tissue is present at the epicenter of the lesion. PMNLs (arrowheads) are present in the injured spinal cord. B, Severe vacuolization of the white matter is observed 24 hr after SCI. C, At 72 hr after SCI, large numbers of macrophages (arrowheads) are observed in the white matter. D, Macrophages (asterisk) and PMNLs (arrowheads) are present at the gray/white interface 72 hr after SCI. The data presented in B–D are from regions of the spinal cord adjacent to the lesion areas. All micrographs are shown at 200× magnification.
Fig. 2.
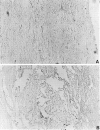
Controls for immunohistochemistry, using sections from sham-operated and SCI animals. A, Spinal cords from sham-operated control animals were stained for activated NF-κB, using DAB immunohistochemistry. Activated NF-κB was not observed in control animals (200× magnification). B, Isotype control for immunohistochemistry, using sections from the lesion epicenter. The primary α-p65 mAb was omitted and replaced with a mouse IgG3 isotype control antibody. No specific staining was observed.
Fig. 3.
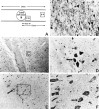
DAB immunohistochemical detection of activated NF-κB 24 hr after traumatic SCI. Activated NF-κB was observed both rostral and caudal to the lesion epicenter throughout the length of the 24 mm sections. A, Schematic diagram describing the expression pattern of activated NF-κB rostral (right side) to the lesion epicenter. B, NF-κB activation was observed within the lesion epicenter (200× magnification; arrowheads). C, Immunohistochemical detection of activated NF-κB within 4 mm of the lesion epicenter (100× magnification). D, Higher magnification (400×) of boxed inset in_C_. E, Activated NF-κB was detected 12 mm away from the lesion epicenter (100× magnification).F, Higher magnification (400×) of the boxed inset in E. Cells positive for NF-κB immunoreactivity have the characteristic size and morphology of neurons (arrows). In C–F, cells that do not have the morphological characteristics of neurons are also positive for activated NF-κB (asterisks).
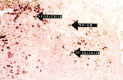
Fig. 4.
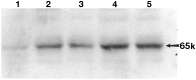
Western blot demonstrating the temporal expression of activated NF-κB after traumatic SCI. Lane 1, Sham-operated control; lane 2, 0.5 hr; lane 3, 1.5 hr; lane 4, 24 hr; and lane 5, 72 hr postinjury. The arrow points to the position of the 65 kDa activated transcription factor. The antibody used in Western blot analysis was the same as that used in our immunohistochemical studies.
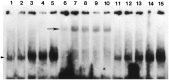
Fig. 5.
EMSA analysis of NF-κB activation after SCI.Lanes 1, 6, 11, Sham-operated control; lanes 2, 7, 12, 0.5 hr; lanes 3, 8, 13, 1.5 hr;lanes 4, 9, 14, 24 hr; and lanes 5, 10, 15, 72 hr postinjury. In lanes 1–5 and_lanes 11–15_ there is a prominent band that interacts with our NF-κB oligonucleotide (arrowhead). Supershift experiments with anti-p65 demonstrate that the protein complex interacting with the NF-κB oligonucleotide contains the p65 subunit (lanes 6–10). When a nonspecific antibody (STAT-1) was used in our supershift experiments, there was no change in the migration pattern of the bands (lanes 11–15).
Fig. 6.
Colocalization of NF-κB immunoreactivity with macrophages/microglia after SCI, using a double immunohistochemical staining procedure 72 hr after SCI. Macrophages/microglia were identified by using an antibody specific for CD11b and by the brown reaction product characteristic of DAB immunohistochemistry. NF-κB immunoreactivity was colocalized with macrophages/microglia (arrows) in the lesion epicenter and adjacent tissue (200× magnification). Cells expressing CD11b and activated NF-κB stained a dark purple or black.
Fig. 7.
Colocalization of activated NF-κB with the NF-κB target gene product iNOS 72 hr after SCI. NF-κB immunoreactivity was visualized by using DAB immunohistochemistry in the first staining reaction. iNOS was visualized by using TrueBlue immunohistochemistry in the subsequent reaction. A, iNOS immunoreactivity was colocalized with NF-κB in non-neuronal cells in the lesion epicenter (200× magnification). B, Sections were stained for iNOS immunoreactivity, using TrueBlue immunohistochemistry; cells with the morphological appearance of neurons expressed iNOS immunoreactivity (200× magnification).C, Colocalization of iNOS with activated NF-κB in neurons results in a rich purple (200× magnification).
Similar articles
- Early nuclear factor-kappaB activation and inducible nitric oxide synthase expression in injured spinal cord neurons correlating with a diffuse reduction of constitutive nitric oxide synthase activity.
Miscusi M, Ebner F, Ceccariglia S, Menegazzi M, Mariotto S, Berra L, Del Fa A, Gangitano C, Lauretti L, Missori P, Delfini R, Suzuki H. Miscusi M, et al. J Neurosurg Spine. 2006 Jun;4(6):485-93. doi: 10.3171/spi.2006.4.6.485. J Neurosurg Spine. 2006. PMID: 16776360 - Inhibition of the nuclear factor-kappaB activation with pyrrolidine dithiocarbamate attenuating inflammation and oxidative stress after experimental spinal cord trauma in rats.
La Rosa G, Cardali S, Genovese T, Conti A, Di Paola R, La Torre D, Cacciola F, Cuzzocrea S. La Rosa G, et al. J Neurosurg Spine. 2004 Oct;1(3):311-21. doi: 10.3171/spi.2004.1.3.0311. J Neurosurg Spine. 2004. PMID: 15478370 - MiR-100 suppresses inflammatory activation of microglia and neuronal apoptosis following spinal cord injury via TLR4/NF-κB pathway.
Li XH, Fu NS, Xing ZM. Li XH, et al. Eur Rev Med Pharmacol Sci. 2019 Oct;23(20):8713-8720. doi: 10.26355/eurrev_201910_19265. Eur Rev Med Pharmacol Sci. 2019. PMID: 31696457 - The NF-κB Pathway: a Focus on Inflammatory Responses in Spinal Cord Injury.
Ding Y, Chen Q. Ding Y, et al. Mol Neurobiol. 2023 Sep;60(9):5292-5308. doi: 10.1007/s12035-023-03411-x. Epub 2023 Jun 7. Mol Neurobiol. 2023. PMID: 37286724 Review. - Current Agents and Related Therapeutic Targets for Inflammation After Acute Traumatic Spinal Cord Injury.
Jorge A, Taylor T, Agarwal N, Hamilton DK. Jorge A, et al. World Neurosurg. 2019 Dec;132:138-147. doi: 10.1016/j.wneu.2019.08.108. Epub 2019 Aug 27. World Neurosurg. 2019. PMID: 31470153 Review.
Cited by
- Neuroprotection and its molecular mechanism following spinal cord injury.
Liu NK, Xu XM. Liu NK, et al. Neural Regen Res. 2012 Sep 15;7(26):2051-62. doi: 10.3969/j.issn.1673-5374.2012.26.007. Neural Regen Res. 2012. PMID: 25624837 Free PMC article. Review. - Matching Diabetes and Alcoholism: Oxidative Stress, Inflammation, and Neurogenesis Are Commonly Involved.
Barcia JM, Flores-Bellver M, Muriach M, Sancho-Pelluz J, Lopez-Malo D, Urdaneta AC, Martinez-Gil N, Atienzar-Aroca S, Romero FJ. Barcia JM, et al. Mediators Inflamm. 2015;2015:624287. doi: 10.1155/2015/624287. Epub 2015 May 7. Mediators Inflamm. 2015. PMID: 26063976 Free PMC article. Review. - Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects.
Karimi-Abdolrezaee S, Billakanti R. Karimi-Abdolrezaee S, et al. Mol Neurobiol. 2012 Oct;46(2):251-64. doi: 10.1007/s12035-012-8287-4. Epub 2012 Jun 9. Mol Neurobiol. 2012. PMID: 22684804 Review. - Molecular basis of vascular events following spinal cord injury.
Sinescu C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M, Burnei G, Strambu V, Popa C. Sinescu C, et al. J Med Life. 2010 Jul-Sep;3(3):254-61. J Med Life. 2010. PMID: 20945816 Free PMC article. Review. - NF-kappaB in neuronal plasticity and neurodegenerative disorders.
Mattson MP, Camandola S. Mattson MP, et al. J Clin Invest. 2001 Feb;107(3):247-54. doi: 10.1172/JCI11916. J Clin Invest. 2001. PMID: 11160145 Free PMC article. Review. No abstract available.
References
- Adams J, Collaco-Moraes Y, de Belleroche J. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J Neurochem. 1996;66:6–13. - PubMed
- Baeuerle PA. The inducible transcription activator NF-κB: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63–80. - PubMed
- Baeuerle PA, Baltimore D. NF-kB: ten years after. Cell. 1996;87:13–20. - PubMed
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical