Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins - PubMed (original) (raw)
Comparative Study
Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins
Y Sun et al. J Neurosci. 1998.
Abstract
The epsilon4 allele of apolipoprotein E (apoE) is associated with increased risk for Alzheimer's disease (AD) and poor outcome after brain injury. In the CNS, apoE is expressed by glia, predominantly astrocytes. To define the potential biological functions of different human apoE isoforms produced within the brain, transgenic mice were generated in which human apoE3 and apoE4 expression is under control of the astrocyte-specific glial fibrillary acidic protein (GFAP) promoter. These animals were then bred back to apoE knock-out mice. Human apoE protein is found within astrocytes and the neuropil throughout development and into the adult period, as assessed by immunocytochemistry and immunoblot analysis in several GFAP-apoE3 and E4 lines. Cultured astrocytes from these mice secrete apoE3 and apoE4 in lipoproteins that are high-density lipoprotein-like in size. When primary hippocampal neurons are grown in the presence of astrocyte monolayers derived from these transgenic mice, there is significantly greater neurite outgrowth from neurons grown in the presence of apoE3-secreting astrocytes compared with apoE4-secreting or apoE knock-out astrocytes. These effects are not dependent on direct astrocyte-neuron contact and appear to require the low-density lipoprotein receptor-related protein. These data suggest that astrocyte-secreted, apoE3-containing lipoproteins have different biological effects than apoE4-containing lipoproteins. In addition to providing information regarding the role of astrocyte-secreted apoE lipoproteins in the normal brain, these animals will also be useful in models of both AD and CNS injury.
Figures
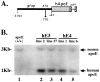
Fig. 1.
GFAP-apoE construct used to generate transgenic mice expressing human apoE3 and apoE4 in the brain and Southern blot analysis for apoE in founder mice. A, Human apoE3 and apoE4 cDNAs (hApoE) were subcloned behind a human glial fibrillary acidic protein (gfap) promoter described by Brenner et al. (1994). B, Southern blot analysis using a 32P-labeled cDNA probe to mouse apoE reveals the presence of human (∼1 kb) and mouse apoE (∼3 kb) DNA in different apoE3 (hE3) and apoE4 (hE4) transgenic lines. Copy number is variable from line to line.
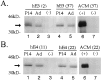
Fig. 2.
Western blot analysis of human apoE in forebrain lysates and concentrated astrocyte-conditioned media (ACM) from transgenic mice expressing apoE3 (hE3; A) or apoE4 (hE4;B). In forebrain samples, 50 μg of detergent-soluble protein was loaded per lane. Human apoE protein is detected with goat anti-human apoE antibody in the P14 and adult (Ad) brains of hE3 (lines 2 and 37) and hE4(lines 11 and 22) transgenic mice but not in nontransgenic (−) apoE knock-out littermates. Astrocytes derived from transgenic (+) animals (hE3, line 37; hE4, line 22) and from nontransgenic (−) apoE knock-out littermates were cultured until confluent and washed, and serum-free media were collected for 48 hr. Media were concentrated 50-fold, and 3 μl was loaded per lane. Human apoE is detected in the serum-free ACM from GFAP-apoE3 line 37 and GFAP-apoE4 line 22 but not in the media of astrocytes derived from nontransgenic apoE knock-out littermates. _Arrow_indicates position of apoE.
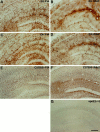
Fig. 3.
ApoE immunoreactivity is present in the brain of GFAP-apoE3 and GFAP-apoE4 mice. GFAP-apoE3 line 37 (A, B) and GFAP-apoE4 line 22 (C, D) mice on a mouse apoE−/− background were immunostained with a goat anti-apoE antibody. There was strong staining of cells, which by morphology appear to be astrocytes in the hippocampus in both P14 (A, C) and adult (B, D) mice. In addition to staining in glial cell bodies and their processes, apoE-IR also appears to be present in the neuropil. Qualitatively similar apoE-IR is seen in cells that appear to be glial in C57Bl6 apoE+/+ mice in both P14 (E) and adult animals (F). ApoE-IR is not observed in the hippocampus of an apoE−/− adult mouse (G). Scale bar, 130 μm.
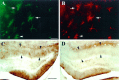
Fig. 4.
GFAP and human apoE are co-localized, and apoE immunoreactivity is increased in regions of denervation in GFAP-apoE transgenic mice. A section through the hippocampus of an adult GFAP-apoE3 line 37 mouse was stained with a rat anti-GFAP antibody (A, green) and a goat anti-apoE antibody (B, red). Cells that are GFAP-immunoreactive are also immunoreactive for apoE (arrows). Three days after unilateral (right) entorhinal cortex lesion in an adult GFAP-apoE4 line 19 mouse, apoE-IR is clearly increased within astrocytes and the neuropil of the denervated outer molecular layer of the dentate gyrus (C). On the nonlesioned side (D), apoE-IR is present but is not upregulated. In C and D, the dorsal blade of the outer molecular layer is bracketed by arrowheads. Scale bars:A, B, 2 μm; C, D, 100 μm.
Fig. 5.
Western blot analysis of selected fractions from gel filtration chromatography of serum-free conditioned media from transgenic astrocytes expressing apoE3 (E3, line 37) or apoE4 (E4, line 22). Human apoE, as detected with rabbit anti-human apoE antisera, was present in fractions eluting in the size range of plasma HDL. The blot was developed with ECL. Arrows indicate positions of apoE (∼36 kDa) monomer (E3, E4), and (∼72 kDa) dimer (E3).
Fig. 6.
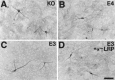
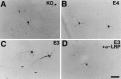
Neurite outgrowth from E19 primary hippocampal neurons is increased when neurons are cultured on the surface of GFAP-apoE3-secreting astrocytes. E19 primary hippocampal neurons (C57Bl6) were plated onto confluent astrocyte monolayers derived from the forebrain of P1 GFAP-apoE3 line 2 (C, D), GFAP-apoE4 line 22 (B), or apoE−/− (A) littermate pups. After 44 hr in culture, MAP-2-IR neurites were on average longer in the presence of the apoE3-secreting astrocytes than in the presence of the apoE4 or apoE−/− astrocytes. The increase in neurite outgrowth seen in the presence of apoE3 was blocked by anti-LRP IgG (D). Scale bar, 26 μm.
Fig. 7.
Neurite outgrowth from E19 primary hippocampal neurons is increased when neurons are cultured in the presence of media derived from GFAP-apoE3-secreting astrocytes. E19 primary hippocampal neurons (C57Bl6) were plated onto poly-
d
-lysine-coated coverslips and after attachment were incubated in the presence (but not in direct contact with) confluent astrocyte monolayers derived from the forebrain of P1 GFAP-apoE3 line 2 (C, D), GFAP-apoE4 line 22 (B), or apoE−/− (A) littermate pups. After 44 hr in culture, neurites, here identified by MAP-2-IR but also identified by phase-contrast microscopy, were on average longer in the presence of the apoE3-secreting astrocytes than in the presence of the apoE4 or apoE−/− astrocytes. The increase in neurite outgrowth seen in the presence of apoE3 was blocked by anti-LRP IgG (D). The tips of two axons are identified with_arrows_ in C. Scale bar, 35 μm.
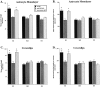
Fig. 8.
Quantification of neurite outgrowth in the presence of human apoE transgenic astrocytes in vitro. Primary hippocampal neurons (C57Bl6) were grown either directly on top of astrocyte monolayers (A, B) or on poly-
d
-lysine-coated coverslips suspended above the astrocyte layer (C, D). Mean values in a representative experiment were obtained from n = 4 wells per condition for each transgenic line (GFAP-E3, line 2; GFAP-apoE4, line 22). In both conditions, mean neurite length and mean neurite length per neuron were significantly greater in the presence of apoE3-expressing astrocytes (E3) compared with apoE4-expressing astrocytes (E4) or those expressing no apoE (KO). In addition, anti-LRP IgG significantly attenuated neurite outgrowth in the presence of apoE3-expressing astrocytes, whereas nonimmune IgG had no effect. Data are presented as mean ± SEM. *p < 0.05 compared with apoE3-secreting astrocytes in the presence of anti-LRP IgG as well as apoE4 and apoE KO astrocytes under all conditions (ANOVA followed by Bonferroni t test).
Similar articles
- Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice.
Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Brecht WJ, et al. J Neurosci. 2004 Mar 10;24(10):2527-34. doi: 10.1523/JNEUROSCI.4315-03.2004. J Neurosci. 2004. PMID: 15014128 Free PMC article. - Lipoproteins produced by ApoE-/- astrocytes infected with adenovirus expressing human ApoE.
Peng D, Song C, Reardon CA, Liao S, Getz GS. Peng D, et al. J Neurochem. 2003 Sep;86(6):1391-402. doi: 10.1046/j.1471-4159.2003.01950.x. J Neurochem. 2003. PMID: 12950448 - Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology.
Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Hartman RE, et al. Exp Neurol. 2001 Aug;170(2):326-44. doi: 10.1006/exnr.2001.7715. Exp Neurol. 2001. PMID: 11476599 - Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-beta deposition in vivo.
Fagan AM, Holtzman DM. Fagan AM, et al. Microsc Res Tech. 2000 Aug 15;50(4):297-304. doi: 10.1002/1097-0029(20000815)50:4<297::AID-JEMT9>3.0.CO;2-C. Microsc Res Tech. 2000. PMID: 10936884 Review. - Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology.
Baum L, Chen L, Ng HK, Pang CP. Baum L, et al. Microsc Res Tech. 2000 Aug 15;50(4):278-81. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10936880 Review.
Cited by
- Androgens protect against apolipoprotein E4-induced cognitive deficits.
Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Raber J, et al. J Neurosci. 2002 Jun 15;22(12):5204-9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. J Neurosci. 2002. PMID: 12077215 Free PMC article. - ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-beta pathology in a mouse model of Alzheimer's disease-like cerebral amyloidosis.
Fagan AM, Christopher E, Taylor JW, Parsadanian M, Spinner M, Watson M, Fryer JD, Wahrle S, Bales KR, Paul SM, Holtzman DM. Fagan AM, et al. Am J Pathol. 2004 Oct;165(4):1413-22. doi: 10.1016/s0002-9440(10)63399-8. Am J Pathol. 2004. PMID: 15466405 Free PMC article. - Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation.
Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ. Manelli AM, et al. Neurobiol Aging. 2007 Aug;28(8):1139-47. doi: 10.1016/j.neurobiolaging.2006.05.024. Epub 2006 Jul 11. Neurobiol Aging. 2007. PMID: 16837105 Free PMC article. - Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease.
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Holtzman DM, et al. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2892-7. doi: 10.1073/pnas.050004797. Proc Natl Acad Sci U S A. 2000. PMID: 10694577 Free PMC article.
References
- Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD. ApoE genotype and survival from intracerebral hemorrhage. Lancet. 1995;346:575. - PubMed
- Barger SW, Mattson MP. Isoform-specific modulation by apolipoprotein E of the activities of secreted β-amyloid precursor protein. J Neurochem. 1997;69:60–67. - PubMed
- Battey F, Gafvels ME, Fitzgerald DJ, Argraves WS, Chappell DA, Strauss JF, Strickland DK. The 39 kDa receptor associated protein regulates ligand binding by the very low density lipoprotein receptor. J Biol Chem. 1994;269:23268–23273. - PubMed
- Bayles KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. - PubMed
- Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of aplipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG013956/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- AG13956/AG/NIA NIH HHS/United States
- R37 AG013956/AG/NIA NIH HHS/United States
- AG05681/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous