Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity - PubMed (original) (raw)
. 1998 Apr 20;187(8):1215-24.
doi: 10.1084/jem.187.8.1215.
B Luckow, P J Nelson, J Cihak, G Simmons, P R Clapham, N Signoret, M Marsh, M Stangassinger, F Borlat, T N Wells, D Schlöndorff, A E Proudfoot
Affiliations
- PMID: 9547333
- PMCID: PMC2212227
- DOI: 10.1084/jem.187.8.1215
Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity
M Mack et al. J Exp Med. 1998.
Abstract
CCR5, a chemokine receptor expressed on T cells and macrophages, is the principal coreceptor for M-tropic HIV-1 strains. Recently, we described an NH2-terminal modification of the CCR5 ligand regulated on activation, normal T cell expressed and secreted (RANTES), aminooxypentane-RANTES (AOP-RANTES), that showed potent inhibition of macrophage infection by HIV-1 under conditions where RANTES was barely effective. To investigate the mechanism of AOP-RANTES inhibition of HIV infectivity we examined the surface expression of CCR5 using a monoclonal anti-CCR5 antibody, MC-1. We demonstrate that AOP-RANTES rapidly caused >90% decrease in cell surface expression of CCR5 on lymphocytes, monocytes/ macrophages, and CCR5 transfected Chinese hamster ovary (CHO) cells. RANTES also caused a loss of cell surface CCR5, although its effect was less than with AOP-RANTES. Significantly, AOP-RANTES inhibited recycling of internalized CCR5 to the cell surface, whereas RANTES did not. When peripheral blood mononuclear cells are cultured for prolonged periods of time in the presence of RANTES, CCR5 expression is comparable to that seen on cells treated with control medium, whereas there is no CCR5 surface expression on cells cultured in the presence of AOP-RANTES. Immunofluorescence indicated that both AOP-RANTES and RANTES induced downmodulation of cell surface CCR5, and that the receptor was redistributed into endocytic organelles containing the transferrin receptor. When RANTES was removed, the internalized receptor was recycled to the cell surface; however, the receptor internalized in the presence of AOP-RANTES was retained in endosomes. Using human osteosarcoma (GHOST) 34/CCR5 cells, the potency of AOP-RANTES and RANTES to inhibit infection by the M-tropic HIV-1 strain, SF 162, correlated with the degree of downregulation of CCR5 induced by the two chemokines. These differences between AOP-RANTES and RANTES in their effect on receptor downregulation and recycling suggest a mechanism for the potent inhibition of HIV infection by AOP-RANTES. Moreover, these results support the notion that receptor internalization and inhibition of receptor recycling present new targets for therapeutic agents to prevent HIV infection.
Figures
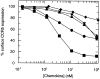
Figure 1
Downregulation of CCR5 from the surface of stably transfected CHO cells. CHO-CCR5 cells were incubated for 30 min at 37°C with the concentrations indicated of the different chemokines: RANTES (•), MIP-1α (▴), MIP-1β (▾), AOP-RANTES (▪), and Met-RANTES (♦). Surface CCR5 was detected with a monoclonal CCR5 antibody (MC-1) and analyzed by flow cytometry. No downregulation was observed with monocyte chemotactic protein (MCP)-1, MCP-2, Gro-α, and SDF-1α at a concentration of 100 nM (data not shown).
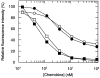
Figure 2
Downregulation of CCR5 on lymphocytes (open symbols) and monocytes (solid symbols). PBMCs were incubated with various concentrations of RANTES (circles) and AOP-RANTES (squares) for 30 min at 37°C, labeled with a monoclonal CCR5 antibody (MC-1), and analyzed as described. It is noteworthy that PBMCs were cultured for 24 h at 37°C in RPMI with 10% FCS. This procedure strongly induces expression of CCR5 on monocytes, whereas expression on lymphocytes was unchanged (data not shown).
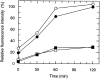
Figure 3
Recycling of CCR5 on lymphocytes (open symbols) and monocytes (closed symbols) after downregulation with RANTES (circles) and AOP-RANTES (squares). PBMCs were first incubated for 30 min at 37°C with 100 nM RANTES or AOP-RANTES. After downregulation, the chemokines were removed by four washing steps with medium, and cells were further cultured in medium at 37°C for various periods of time and analyzed for CCR5 expression as described above.
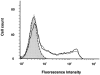
Figure 4
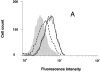
Histogram analysis showing the surface expression of CCR5 on lymphocytes after 12 d of incubation with 100 nM RANTES and AOP-RANTES. CCR5 expression was measured as described in Fig. 2. Incubation with medium (solid line), 100 nM RANTES (dotted line), or 100 nM AOP-RANTES (dashed line). Shaded area, secondary antibody alone.
Figure 5
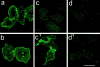
Downmodulation and recycling of CCR5. CHO/CCR5 cells were treated with medium (a) or medium containing either RANTES (250 nM; c and c′ ), AOP-RANTES (125 nM; d and d′ ), or Met-RANTES (250 nM; b) for 30 min at 37°C. The cells were then cooled on ice, washed extensively in cold medium, and either held on ice or reincubated at 37°C for a further 60 min in medium (c′ and d′ ). Subsequently, all cells were fixed and labeled with the anti-CCR5 mAb MC1. Bar, 40 mm.
Figure 6
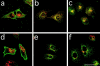
Intracellular localization of downmodulated CCR5. CHO/CCR5 were treated with medium (a and d), medium with RANTES (250 nM; b and e), or AOP-RANTES (125 nM; c and f ) for 1 h at 37°C. After fixation and permeabilization, the cells were costained for CCR5 (green) and TfR (a, b, and c; red) or a lysosomal marker Lgp-B (d, e, and f; red). Scale bar, 40 mm.
Figure 7
The induction of calcium mobilization induced by RANTES (•), AOP-RANTES (▪), and Met-RANTES (♦) in CHO/CCR5 cells. Calcium mobilization was determined as previously described (25) using 2 × 106 Fura-2–loaded cells for each measurement, with the concentration of chemokine as indicated.
Figure 8
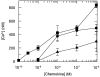
Downregulation of CCR5 from the surface of GHOST 34/ CCR5 cells and the inhibition of their infection by the M-tropic HIV-1 strain SF 162 by RANTES and AOP-RANTES. (A) Downregulation of CCR5 induced by 100 nM RANTES. The medium control is shown by the solid line and surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (B) Downregulation of CCR5 induced by 100 nM AOP-RANTES. The medium control is shown by the solid line and the surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (C) The inhibition of infection of GHOST 34/CCR5 cells by SF 162 by increasing concentrations of RANTES (hatched bars) and AOP-RANTES (solid bars).
Figure 8
Downregulation of CCR5 from the surface of GHOST 34/ CCR5 cells and the inhibition of their infection by the M-tropic HIV-1 strain SF 162 by RANTES and AOP-RANTES. (A) Downregulation of CCR5 induced by 100 nM RANTES. The medium control is shown by the solid line and surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (B) Downregulation of CCR5 induced by 100 nM AOP-RANTES. The medium control is shown by the solid line and the surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (C) The inhibition of infection of GHOST 34/CCR5 cells by SF 162 by increasing concentrations of RANTES (hatched bars) and AOP-RANTES (solid bars).
Figure 8
Downregulation of CCR5 from the surface of GHOST 34/ CCR5 cells and the inhibition of their infection by the M-tropic HIV-1 strain SF 162 by RANTES and AOP-RANTES. (A) Downregulation of CCR5 induced by 100 nM RANTES. The medium control is shown by the solid line and surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (B) Downregulation of CCR5 induced by 100 nM AOP-RANTES. The medium control is shown by the solid line and the surface expression of CCR5 after 30 min by the dotted line and after 3.5 h by the dashed line. (C) The inhibition of infection of GHOST 34/CCR5 cells by SF 162 by increasing concentrations of RANTES (hatched bars) and AOP-RANTES (solid bars).
Similar articles
- Endocytosis and recycling of the HIV coreceptor CCR5.
Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Signoret N, et al. J Cell Biol. 2000 Dec 11;151(6):1281-94. doi: 10.1083/jcb.151.6.1281. J Cell Biol. 2000. PMID: 11121442 Free PMC article. - Variable sensitivity of CCR5-tropic human immunodeficiency virus type 1 isolates to inhibition by RANTES analogs.
Torre VS, Marozsan AJ, Albright JL, Collins KR, Hartley O, Offord RE, Quiñones-Mateu ME, Arts EJ. Torre VS, et al. J Virol. 2000 May;74(10):4868-76. doi: 10.1128/jvi.74.10.4868-4876.2000. J Virol. 2000. PMID: 10775626 Free PMC article. - Mechanisms involved in stimulation of human immunodeficiency virus type 1 replication by aminooxypentane RANTES.
Marozsan AJ, Torre VS, Johnson M, Ball SC, Cross JV, Templeton DJ, Quiñones-Mateu ME, Offord RE, Arts EJ. Marozsan AJ, et al. J Virol. 2001 Sep;75(18):8624-38. doi: 10.1128/jvi.75.18.8624-8638.2001. J Virol. 2001. PMID: 11507208 Free PMC article. - Chemokine-induced phosphorylation of CC chemokine receptor 5 (CCR5).
Olbrich H, Proudfoot AE, Oppermann M. Olbrich H, et al. J Leukoc Biol. 1999 Mar;65(3):281-5. doi: 10.1002/jlb.65.3.281. J Leukoc Biol. 1999. PMID: 10080528 Review. - Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands.
Simmons G, Reeves JD, Hibbitts S, Stine JT, Gray PW, Proudfoot AE, Clapham PR. Simmons G, et al. Immunol Rev. 2000 Oct;177:112-26. doi: 10.1034/j.1600-065x.2000.17719.x. Immunol Rev. 2000. PMID: 11138769 Review.
Cited by
- Endocytosis and recycling of the HIV coreceptor CCR5.
Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Signoret N, et al. J Cell Biol. 2000 Dec 11;151(6):1281-94. doi: 10.1083/jcb.151.6.1281. J Cell Biol. 2000. PMID: 11121442 Free PMC article. - Endogenous Peptide Inhibitors of HIV Entry.
Harms M, Hayn M, Zech F, Kirchhoff F, Münch J. Harms M, et al. Adv Exp Med Biol. 2022;1366:65-85. doi: 10.1007/978-981-16-8702-0_5. Adv Exp Med Biol. 2022. PMID: 35412135 - Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors.
Hartley O, Gaertner H, Wilken J, Thompson D, Fish R, Ramos A, Pastore C, Dufour B, Cerini F, Melotti A, Heveker N, Picard L, Alizon M, Mosier D, Kent S, Offord R. Hartley O, et al. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16460-5. doi: 10.1073/pnas.0404802101. Epub 2004 Nov 15. Proc Natl Acad Sci U S A. 2004. PMID: 15545608 Free PMC article. - Circulating human CD4 and CD8 T cells do not have large intracellular pools of CCR5.
Pilch-Cooper HA, Sieg SF, Hope TJ, Koons A, Escola JM, Offord R, Veazey RS, Mosier DE, Clagett B, Medvik K, Jadlowsky JK, Chance MR, Kiselar JG, Hoxie JA, Collman RG, Riddick NE, Mercanti V, Hartley O, Lederman MM. Pilch-Cooper HA, et al. Blood. 2011 Jul 28;118(4):1015-9. doi: 10.1182/blood-2010-05-282509. Epub 2010 Nov 10. Blood. 2011. PMID: 21068438 Free PMC article. - Enhanced inhibition of human immunodeficiency virus type 1 by Met-stromal-derived factor 1beta correlates with down-modulation of CXCR4.
Yang OO, Swanberg SL, Lu Z, Dziejman M, McCoy J, Luster AD, Walker BD, Herrmann SH. Yang OO, et al. J Virol. 1999 Jun;73(6):4582-9. doi: 10.1128/JVI.73.6.4582-4589.1999. J Virol. 1999. PMID: 10233917 Free PMC article.
References
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major coreceptor for primary isolates of HIV-1. Nature. 1996;381:661–666. - PubMed
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. - PubMed
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein–coupled receptor. Science. 1996;272:872–877. - PubMed
- Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical