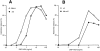Involvement of Bruton's tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production - PubMed (original) (raw)
. 1998 Apr 20;187(8):1235-47.
doi: 10.1084/jem.187.8.1235.
Y Kawakami, N Inagaki, C S Lantz, T Kitamura, W N Khan, M Maeda-Yamamoto, T Miura, W Han, S E Hartman, L Yao, H Nagai, A E Goldfeld, F W Alt, S J Galli, O N Witte, T Kawakami
Affiliations
- PMID: 9547335
- PMCID: PMC2212237
- DOI: 10.1084/jem.187.8.1235
Involvement of Bruton's tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production
D Hata et al. J Exp Med. 1998.
Abstract
We investigated the role of Bruton's tyrosine kinase (Btk) in FcepsilonRI-dependent activation of mouse mast cells, using xid and btk null mutant mice. Unlike B cell development, mast cell development is apparently normal in these btk mutant mice. However, mast cells derived from these mice exhibited significant abnormalities in FcepsilonRI-dependent function. xid mice primed with anti-dinitrophenyl monoclonal IgE antibody exhibited mildly diminished early-phase and severely blunted late-phase anaphylactic reactions in response to antigen challenge in vivo. Consistent with this finding, cultured mast cells derived from the bone marrow cells of xid or btk null mice exhibited mild impairments in degranulation, and more profound defects in the production of several cytokines, upon FcepsilonRI cross-linking. Moreover, the transcriptional activities of these cytokine genes were severely reduced in FcepsilonRI-stimulated btk mutant mast cells. The specificity of these effects of btk mutations was confirmed by the improvement in the ability of btk mutant mast cells to degranulate and to secrete cytokines after the retroviral transfer of wild-type btk cDNA, but not of vector or kinase-dead btk cDNA. Retroviral transfer of Emt (= Itk/Tsk), Btk's closest relative, also partially improved the ability of btk mutant mast cells to secrete mediators. Taken together, these results demonstrate an important role for Btk in the full expression of FcepsilonRI signal transduction in mast cells.
Figures
Figure 1
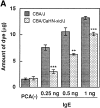
PCA reactions in CBA/J (wt) and CBA/CaHN-xid/J (xid) mice. (A) Mice were left unsensitized [_PCA(-)_] or sensitized by intradermal injection of the indicated amounts of anti-DNP IgE in 10 μl solution at the ear 24 h before antigen challenge. Mice were stimulated by intravenous injection of DNP8.7-BSA and Evans blue dye for 30 min, and then the amount of extravasated Evans blue dye in the IgE-injected and control ears was measured as previously described (41). Statistical significance is indicated by ** (P <0.01) and *** (P <0.001). (B) Mice were left unsensitized or sensitized by intravenous injection of anti-DNP monoclonal IgE 24 h before antigen challenge. 25 μl of 0.75% dinitrofluorobenzene was applied on both sides of the ear, and the ear thickness was measured (42) at the indicated intervals. In C, anti–TNF-α antibody (aTNF, 80,000 U/0.2 ml/mouse) or normal rabbit serum (NRS, 0.2 ml/mouse) was intravenously injected just before antigen challenge in wt mice which had been sensitized with IgE and challenged with antigen (hapten) epicutaneously as in B; ear thickness was measured 24 h after antigen challenge.
Figure 1
PCA reactions in CBA/J (wt) and CBA/CaHN-xid/J (xid) mice. (A) Mice were left unsensitized [_PCA(-)_] or sensitized by intradermal injection of the indicated amounts of anti-DNP IgE in 10 μl solution at the ear 24 h before antigen challenge. Mice were stimulated by intravenous injection of DNP8.7-BSA and Evans blue dye for 30 min, and then the amount of extravasated Evans blue dye in the IgE-injected and control ears was measured as previously described (41). Statistical significance is indicated by ** (P <0.01) and *** (P <0.001). (B) Mice were left unsensitized or sensitized by intravenous injection of anti-DNP monoclonal IgE 24 h before antigen challenge. 25 μl of 0.75% dinitrofluorobenzene was applied on both sides of the ear, and the ear thickness was measured (42) at the indicated intervals. In C, anti–TNF-α antibody (aTNF, 80,000 U/0.2 ml/mouse) or normal rabbit serum (NRS, 0.2 ml/mouse) was intravenously injected just before antigen challenge in wt mice which had been sensitized with IgE and challenged with antigen (hapten) epicutaneously as in B; ear thickness was measured 24 h after antigen challenge.
Figure 1
PCA reactions in CBA/J (wt) and CBA/CaHN-xid/J (xid) mice. (A) Mice were left unsensitized [_PCA(-)_] or sensitized by intradermal injection of the indicated amounts of anti-DNP IgE in 10 μl solution at the ear 24 h before antigen challenge. Mice were stimulated by intravenous injection of DNP8.7-BSA and Evans blue dye for 30 min, and then the amount of extravasated Evans blue dye in the IgE-injected and control ears was measured as previously described (41). Statistical significance is indicated by ** (P <0.01) and *** (P <0.001). (B) Mice were left unsensitized or sensitized by intravenous injection of anti-DNP monoclonal IgE 24 h before antigen challenge. 25 μl of 0.75% dinitrofluorobenzene was applied on both sides of the ear, and the ear thickness was measured (42) at the indicated intervals. In C, anti–TNF-α antibody (aTNF, 80,000 U/0.2 ml/mouse) or normal rabbit serum (NRS, 0.2 ml/mouse) was intravenously injected just before antigen challenge in wt mice which had been sensitized with IgE and challenged with antigen (hapten) epicutaneously as in B; ear thickness was measured 24 h after antigen challenge.
Figure 2
FcεRI-induced histamine release and cytokine secretion from wt and _xid_-BMMCs. BMMCs were sensitized by overnight incubation with 1 μg/ml anti-DNP monoclonal IgE antibody. Cells resuspended in Tyrode solution (A and B) or culture media (C) were stimulated by the indicated concentrations of DNP-HSA for 45 min (A and B) or 24 h (C). Histamine released into the buffer was measured by an automatic fluorometric assay (A and B). Cytokines secreted into the culture media during 24 h were measured by ELISA (C). The results of histamine release are mean values of duplicates per point, with SEM <5% of the mean. The results shown in A for _xid_-BMMCs and their corresponding _wt-_BMMCs are representative of those obtained in more than five experiments. The results shown in B are representative of those from two experiments. Cytokine measurements shown in C were performed in triplicate and mean values are shown, with SEM <10% of the mean. The results in C are representative of those from at least seven experiments.
Figure 2
FcεRI-induced histamine release and cytokine secretion from wt and _xid_-BMMCs. BMMCs were sensitized by overnight incubation with 1 μg/ml anti-DNP monoclonal IgE antibody. Cells resuspended in Tyrode solution (A and B) or culture media (C) were stimulated by the indicated concentrations of DNP-HSA for 45 min (A and B) or 24 h (C). Histamine released into the buffer was measured by an automatic fluorometric assay (A and B). Cytokines secreted into the culture media during 24 h were measured by ELISA (C). The results of histamine release are mean values of duplicates per point, with SEM <5% of the mean. The results shown in A for _xid_-BMMCs and their corresponding _wt-_BMMCs are representative of those obtained in more than five experiments. The results shown in B are representative of those from two experiments. Cytokine measurements shown in C were performed in triplicate and mean values are shown, with SEM <10% of the mean. The results in C are representative of those from at least seven experiments.
Figure 3
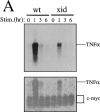
Analysis of TNF-α mRNA and protein in wt and xid mast cells. (A) Northern blot analysis for TNF-α mRNA of total cellular RNAs from immunologically stimulated BMMCs (top). The same blot was reprobed with c-myc cDNA (bottom). FcεRI cross-linking did not change c-myc mRNA levels. Because the blot was not stripped before reprobing, the TNF-α mRNA signals were also detected by the second probing. (B) Immunoblotting analysis of membrane-bound TNF-α (pro-TNFα) in _wt_- and _xid_-BMMCs. Cells were immunologically stimulated via FcεRI for the indicated times. Detergent lysates were analyzed by Western blotting with polyclonal anti–TNF-α antibodies. Positions of molecular mass standards (in kilodaltons) and pro–TNF-α are indicated on the left and right, respectively.
Figure 3
Analysis of TNF-α mRNA and protein in wt and xid mast cells. (A) Northern blot analysis for TNF-α mRNA of total cellular RNAs from immunologically stimulated BMMCs (top). The same blot was reprobed with c-myc cDNA (bottom). FcεRI cross-linking did not change c-myc mRNA levels. Because the blot was not stripped before reprobing, the TNF-α mRNA signals were also detected by the second probing. (B) Immunoblotting analysis of membrane-bound TNF-α (pro-TNFα) in _wt_- and _xid_-BMMCs. Cells were immunologically stimulated via FcεRI for the indicated times. Detergent lysates were analyzed by Western blotting with polyclonal anti–TNF-α antibodies. Positions of molecular mass standards (in kilodaltons) and pro–TNF-α are indicated on the left and right, respectively.
Figure 4
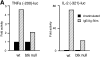
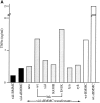
Transcriptional activity of luciferase reporter constructs in wt mast cells or btk null mast cells reconstituted with wt or K430R btk cDNAs. Promoter reporter constructs, IL-2 (−321)–luc, TNF-α (−200)–luc, NFAT–luc, NFκB–luc, or c-_fos_–luc, were transfected into wt or btk null mast cells (A) or btk null mast cells reconstituted with vector, wt btk, or K430R btk retroviruses (B). Luciferase activity was measured in cell lysates that were prepared 8 h after antigen stimulation or mock stimulation. Fold activities in FcεRI-stimulated cells versus unstimulated cells (= 1) are shown. The results shown are representative of those obtained in at least three experiments of each type.
Figure 4
Transcriptional activity of luciferase reporter constructs in wt mast cells or btk null mast cells reconstituted with wt or K430R btk cDNAs. Promoter reporter constructs, IL-2 (−321)–luc, TNF-α (−200)–luc, NFAT–luc, NFκB–luc, or c-_fos_–luc, were transfected into wt or btk null mast cells (A) or btk null mast cells reconstituted with vector, wt btk, or K430R btk retroviruses (B). Luciferase activity was measured in cell lysates that were prepared 8 h after antigen stimulation or mock stimulation. Fold activities in FcεRI-stimulated cells versus unstimulated cells (= 1) are shown. The results shown are representative of those obtained in at least three experiments of each type.
Figure 5
Effects of retroviral transfection of btk cDNAs on the ability of xid or btk null mast cells to produce cytokines or degranulate. (A) _xid_-sBMMCs were infected with the retroviruses encoding wt, kinase-dead (K430R), xid, or E41K mutant btk cDNAs. As controls, _xid_-sBMMCs were also transfected with lyn or syk cDNAs. G418-resistant cells were immunologically stimulated via the FcεRI and the TNF-α secreted during the next 24 h was measured. Levels of TNF-α secreted over 24 h by FcεRI-activated, but nontransfected _xid_-BMMCs, _xid_-sBMMCs, _wt_-BMMCs, and _wt_-sBMMCs, are also shown. The results presented are representative of those obtained in three separate experiments. The TNF-α values of duplicate measurements varied <10%. (B) FcεRI-induced secretion of TNF-α, IL-2, IL-6, and GM-CSF from _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; also shown is cytokine secretion from vector control, wt emt, or kinase-dead (K390R) mutant _emt_-transfected _btk null_–sBMMCs. (C) Histamine release from IgE/antigen (30 ng/ml DNP-HSA)–stimulated _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; results with _wt emt_– or K390R _emt_–transfected cells are also shown. Note that the percentage of histamine release from wt_-sBMMCs, which had been cultured for a total of 7 wk from the start of bone marrow cell culture, is less than that observed in younger cultures of BMMCs (e.g., see Fig. 2_B). In several experiments, 7-wk-old cultures of _wt_-sBMMCs released 10–30% of their cellular content upon FcεRI cross-linking. (D) Expression of Btk and Emt proteins in transfected _xid_-sBMMCs (left) and _btk null_–sBMMCs (center and right). Transfected cells were lysed and analyzed by SDS-PAGE and immunoblotting with anti-BtkC (43) or anti-Emt (16) antibodies.
Figure 5
Effects of retroviral transfection of btk cDNAs on the ability of xid or btk null mast cells to produce cytokines or degranulate. (A) _xid_-sBMMCs were infected with the retroviruses encoding wt, kinase-dead (K430R), xid, or E41K mutant btk cDNAs. As controls, _xid_-sBMMCs were also transfected with lyn or syk cDNAs. G418-resistant cells were immunologically stimulated via the FcεRI and the TNF-α secreted during the next 24 h was measured. Levels of TNF-α secreted over 24 h by FcεRI-activated, but nontransfected _xid_-BMMCs, _xid_-sBMMCs, _wt_-BMMCs, and _wt_-sBMMCs, are also shown. The results presented are representative of those obtained in three separate experiments. The TNF-α values of duplicate measurements varied <10%. (B) FcεRI-induced secretion of TNF-α, IL-2, IL-6, and GM-CSF from _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; also shown is cytokine secretion from vector control, wt emt, or kinase-dead (K390R) mutant _emt_-transfected _btk null_–sBMMCs. (C) Histamine release from IgE/antigen (30 ng/ml DNP-HSA)–stimulated _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; results with _wt emt_– or K390R _emt_–transfected cells are also shown. Note that the percentage of histamine release from wt_-sBMMCs, which had been cultured for a total of 7 wk from the start of bone marrow cell culture, is less than that observed in younger cultures of BMMCs (e.g., see Fig. 2_B). In several experiments, 7-wk-old cultures of _wt_-sBMMCs released 10–30% of their cellular content upon FcεRI cross-linking. (D) Expression of Btk and Emt proteins in transfected _xid_-sBMMCs (left) and _btk null_–sBMMCs (center and right). Transfected cells were lysed and analyzed by SDS-PAGE and immunoblotting with anti-BtkC (43) or anti-Emt (16) antibodies.
Figure 5
Effects of retroviral transfection of btk cDNAs on the ability of xid or btk null mast cells to produce cytokines or degranulate. (A) _xid_-sBMMCs were infected with the retroviruses encoding wt, kinase-dead (K430R), xid, or E41K mutant btk cDNAs. As controls, _xid_-sBMMCs were also transfected with lyn or syk cDNAs. G418-resistant cells were immunologically stimulated via the FcεRI and the TNF-α secreted during the next 24 h was measured. Levels of TNF-α secreted over 24 h by FcεRI-activated, but nontransfected _xid_-BMMCs, _xid_-sBMMCs, _wt_-BMMCs, and _wt_-sBMMCs, are also shown. The results presented are representative of those obtained in three separate experiments. The TNF-α values of duplicate measurements varied <10%. (B) FcεRI-induced secretion of TNF-α, IL-2, IL-6, and GM-CSF from _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; also shown is cytokine secretion from vector control, wt emt, or kinase-dead (K390R) mutant _emt_-transfected _btk null_–sBMMCs. (C) Histamine release from IgE/antigen (30 ng/ml DNP-HSA)–stimulated _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; results with _wt emt_– or K390R _emt_–transfected cells are also shown. Note that the percentage of histamine release from wt_-sBMMCs, which had been cultured for a total of 7 wk from the start of bone marrow cell culture, is less than that observed in younger cultures of BMMCs (e.g., see Fig. 2_B). In several experiments, 7-wk-old cultures of _wt_-sBMMCs released 10–30% of their cellular content upon FcεRI cross-linking. (D) Expression of Btk and Emt proteins in transfected _xid_-sBMMCs (left) and _btk null_–sBMMCs (center and right). Transfected cells were lysed and analyzed by SDS-PAGE and immunoblotting with anti-BtkC (43) or anti-Emt (16) antibodies.
Figure 5
Effects of retroviral transfection of btk cDNAs on the ability of xid or btk null mast cells to produce cytokines or degranulate. (A) _xid_-sBMMCs were infected with the retroviruses encoding wt, kinase-dead (K430R), xid, or E41K mutant btk cDNAs. As controls, _xid_-sBMMCs were also transfected with lyn or syk cDNAs. G418-resistant cells were immunologically stimulated via the FcεRI and the TNF-α secreted during the next 24 h was measured. Levels of TNF-α secreted over 24 h by FcεRI-activated, but nontransfected _xid_-BMMCs, _xid_-sBMMCs, _wt_-BMMCs, and _wt_-sBMMCs, are also shown. The results presented are representative of those obtained in three separate experiments. The TNF-α values of duplicate measurements varied <10%. (B) FcεRI-induced secretion of TNF-α, IL-2, IL-6, and GM-CSF from _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; also shown is cytokine secretion from vector control, wt emt, or kinase-dead (K390R) mutant _emt_-transfected _btk null_–sBMMCs. (C) Histamine release from IgE/antigen (30 ng/ml DNP-HSA)–stimulated _btk null_–sBMMCs transfected with wt, K430R, xid (R28C), SH2 (R307K), or SH3 (P265L) mutant btk cDNAs; results with _wt emt_– or K390R _emt_–transfected cells are also shown. Note that the percentage of histamine release from wt_-sBMMCs, which had been cultured for a total of 7 wk from the start of bone marrow cell culture, is less than that observed in younger cultures of BMMCs (e.g., see Fig. 2_B). In several experiments, 7-wk-old cultures of _wt_-sBMMCs released 10–30% of their cellular content upon FcεRI cross-linking. (D) Expression of Btk and Emt proteins in transfected _xid_-sBMMCs (left) and _btk null_–sBMMCs (center and right). Transfected cells were lysed and analyzed by SDS-PAGE and immunoblotting with anti-BtkC (43) or anti-Emt (16) antibodies.
Figure 6
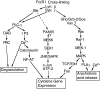
Hypothetical scheme of the roles of Btk in FcεRI-mediated signal transduction. Phosphorylation at Tyr-551 by activated Lyn leads to activation of Btk. Activated Btk in turn may phosphorylate PLC-γ, leading to Ca2+ mobilization. This pathway will activate NFAT as a result of dephosphorylation by calcineurin. Btk also can activate the JNK/SAPK pathway, resulting in the activation of c-Jun and ATF-2 by the phosphorylation of critical residues. Active NFAT, c-Jun and ATF-2 then lead to the transcriptional activation of cytokine genes.
Similar articles
- Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses.
Iyer AS, Morales JL, Huang W, Ojo F, Ning G, Wills E, Baines JD, August A. Iyer AS, et al. J Biol Chem. 2011 Mar 18;286(11):9503-13. doi: 10.1074/jbc.M110.165613. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212279 Free PMC article. - Defective degranulation and calcium mobilization of bone-marrow derived mast cells from Xid and Btk-deficient mice.
Setoguchi R, Kinashi T, Sagara H, Hirosawa K, Takatsu K. Setoguchi R, et al. Immunol Lett. 1998 Dec;64(2-3):109-18. doi: 10.1016/s0165-2478(98)00086-8. Immunol Lett. 1998. PMID: 9870661 - Bruton's tyrosine kinase-mediated interleukin-2 gene activation in mast cells. Dependence on the c-Jun N-terminal kinase activation pathway.
Hata D, Kitaura J, Hartman SE, Kawakami Y, Yokota T, Kawakami T. Hata D, et al. J Biol Chem. 1998 May 1;273(18):10979-87. doi: 10.1074/jbc.273.18.10979. J Biol Chem. 1998. PMID: 9556577 - Functions of Bruton's tyrosine kinase in mast and B cells.
Kawakami Y, Kitaura J, Hata D, Yao L, Kawakami T. Kawakami Y, et al. J Leukoc Biol. 1999 Mar;65(3):286-90. doi: 10.1002/jlb.65.3.286. J Leukoc Biol. 1999. PMID: 10080529 Review. - Tec family kinases: regulation of FcεRI-mediated mast-cell activation.
Ellmeier W, Abramova A, Schebesta A. Ellmeier W, et al. FEBS J. 2011 Jun;278(12):1990-2000. doi: 10.1111/j.1742-4658.2011.08073.x. Epub 2011 Mar 29. FEBS J. 2011. PMID: 21362140 Review.
Cited by
- B cell-dependent T cell responses: IgM antibodies are required to elicit contact sensitivity.
Tsuji RF, Szczepanik M, Kawikova I, Paliwal V, Campos RA, Itakura A, Akahira-Azuma M, Baumgarth N, Herzenberg LA, Askenase PW. Tsuji RF, et al. J Exp Med. 2002 Nov 18;196(10):1277-90. doi: 10.1084/jem.20020649. J Exp Med. 2002. PMID: 12438420 Free PMC article. - Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses.
Iyer AS, Morales JL, Huang W, Ojo F, Ning G, Wills E, Baines JD, August A. Iyer AS, et al. J Biol Chem. 2011 Mar 18;286(11):9503-13. doi: 10.1074/jbc.M110.165613. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212279 Free PMC article. - High-resolution three-dimensional imaging of the rich membrane structures of bone marrow-derived mast cells.
Zink T, Deng Z, Chen H, Yu L, Liu FT, Liu GY. Zink T, et al. Ultramicroscopy. 2008 Dec;109(1):22-31. doi: 10.1016/j.ultramic.2008.07.007. Epub 2008 Aug 6. Ultramicroscopy. 2008. PMID: 18790570 Free PMC article. - Role of Bruton's tyrosine kinase in B cells and malignancies.
Pal Singh S, Dammeijer F, Hendriks RW. Pal Singh S, et al. Mol Cancer. 2018 Feb 19;17(1):57. doi: 10.1186/s12943-018-0779-z. Mol Cancer. 2018. PMID: 29455639 Free PMC article. Review. - The Tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses.
Iyer AS, August A. Iyer AS, et al. J Immunol. 2008 Jun 15;180(12):7869-77. doi: 10.4049/jimmunol.180.12.7869. J Immunol. 2008. PMID: 18523250 Free PMC article.
References
- Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. - PubMed
- Beaven MA, Metzger H. Signal transduction by Fc receptors: the FcεRI case. Immunol Today. 1993;14:222–226. - PubMed
- Ravetch JV, Kinet J-P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. - PubMed
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. - PubMed
- Cambier JC. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol Today. 1995;16:110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI-33617/AI/NIAID NIH HHS/United States
- R01 AI-38348/AI/NIAID NIH HHS/United States
- R01 CA072074/CA/NCI NIH HHS/United States
- R37 AI023990/AI/NIAID NIH HHS/United States
- R37 AI-23990/AI/NIAID NIH HHS/United States
- R01 AI038348/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases