Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates - PubMed (original) (raw)
Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates
H A Bazan et al. J Virol. 1998 May.
Abstract
Coreceptor usage by Envs from diverse primary human immunodeficiency virus type 1 isolates was analyzed by a vaccinia virus-based expression and assay system. Usage of recombinant CCR5 and CXCR4 correlated closely with fusogenicity toward macrophages and T-cell lines expressing endogenous coreceptors. Surprisingly, recombinant CCR3 was utilized by most primary and T-cell-line-adapted Envs. Endogenous CXCR4 in macrophages was functional as a coreceptor.
Figures
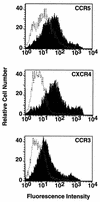
FIG. 1
Surface expression of coreceptors. NIH 3T3 cells were transfected with pSC59-based plasmids containing a synthetic vaccinia virus promoter linked to the indicated coreceptor genes and coinfected with vCB-3 (CD4) and vCB21R-LacZ; control cells were transfected with the empty pSC59 plasmid and infected identically. Following overnight incubation to allow expression of vaccinia virus-encoded proteins, the cells were stained with the corresponding antibodies as follows: for CCR5, rabbit polyclonal antisera against a synthetic peptide representing the CCR5 extracellular N terminus (1:50 dilution) (4); for CXCR4, the 12G5 monoclonal antibody (23 μg/ml) (22), donated by J. Hoxie, University of Pennsylvania; and for CCR3, the 7B11 monoclonal antibody (20 μg/ml) (29), donated by C. Mackay, Leukosite. Detection was achieved with the following secondary antibodies (10 μg/ml; Boehringer Mannheim): for CCR5, fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G; and for CXCR4 and CCR3, fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G. The cells were washed, treated with 1 μg of ethidium bromide homodimer per ml, fixed with 0.1 ml of 4% paraformaldehyde, and analyzed with a FACSCAN flow cytometer (Becton Dickinson, Menlo Park, Calif.). Analyses of forward and side scatter as well as ethidium bromide homodimer fluorescence indicated nearly homogeneous populations of viable cells. Shaded profiles indicate coreceptor-expressing cells; unshaded profiles indicate control cells transfected with the empty pSC59 plasmid. The mean fluorescence intensities with coreceptor-expressing versus control cells were as follows: CCR5, 92 versus 21; CXCR4, 167 versus 4; and CCR3, 57 versus 9. In separate experiments, similar distinctions were seen when coreceptor-expressing cells were stained with either an anticoreceptor monoclonal antibody or an isotype-matched control monoclonal antibody (data not shown).
FIG. 2
Fusogenic activities of each Env with recombinant coreceptors. Target NIH 3T3 cells coexpressing coreceptors and CD4 (and containing the lacZ gene linked to the T7 promoter) as well as control cells lacking coreceptors were prepared as described in the legend for Fig. 1. Effector HeLa cells were transfected with plasmids encoding the indicated Envs and then infected with vP11T7gene1 (T7 polymerase). After overnight incubation to allow recombinant protein expression, cells were mixed and fusion was scored after 3 h. The low background values for each Env obtained with the control target cells expressing CD4 but no coreceptors were subtracted to give the data shown. Error bars indicate the sample standard deviations of the mean values obtained from duplicate samples. OD, optical density.
FIG. 3
(A) Correlation between coreceptor usage profiles and fusion specificities for natural target cells. For each Env, the ratio of the fusion activity with CCR5 versus CXCR4 was calculated from the data shown in Fig. 2 (open triangles); the ratio of the relative fusion activity obtained with macrophage versus Jurkat cell targets was calculated from the data in Table 1 (closed circles). All ratio values below 0.1 or above 10 were grouped together. (B) Functional CXCR4 coreceptor on macrophages. Macrophages coinfected with vTF7-3 (T7 RNA polymerase) and vCB-3 (CD4) were preincubated for 45 min at 37°C without antibody or with 1 mg of preimmune or immune immunoglobulin per ml purified from a rabbit immunized with a peptide representing the extracellular N terminus of CXCR4 (24). Effector HeLa cells were coinfected with vCB21RLacZ and either vCB-41 (LAV Env), vCB-43 (Ba-L Env), or vCB-16 (Unc Env). The Ba-L Env infection was performed with AraC to reduce the fusion activity so that it was comparable to that of the LAV Env; similar results were obtained when the Ba-L Env was expressed without AraC (i.e., no inhibition; data not shown). For the LAV and Ba-L Envs, the minimal values obtained with Unc Env were subtracted and the results are expressed as the percentage of activity obtained in the absence of antibody (set at 100%). Error bars indicate the sample standard deviations of the mean values obtained from duplicate samples.
Similar articles
- Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4.
Cho MW, Lee MK, Carney MC, Berson JF, Doms RW, Martin MA. Cho MW, et al. J Virol. 1998 Mar;72(3):2509-15. doi: 10.1128/JVI.72.3.2509-2515.1998. J Virol. 1998. PMID: 9499115 Free PMC article. - Influence of the CCR2-V64I polymorphism on human immunodeficiency virus type 1 coreceptor activity and on chemokine receptor function of CCR2b, CCR3, CCR5, and CXCR4.
Lee B, Doranz BJ, Rana S, Yi Y, Mellado M, Frade JM, Martinez-A C, O'Brien SJ, Dean M, Collman RG, Doms RW. Lee B, et al. J Virol. 1998 Sep;72(9):7450-8. doi: 10.1128/JVI.72.9.7450-7458.1998. J Virol. 1998. PMID: 9696841 Free PMC article. - Exclusion of HIV coreceptors CXCR4, CCR5, and CCR3 from the HIV envelope.
Lallos LB, Laal S, Hoxie JA, Zolla-Pazner S, Bandres JC. Lallos LB, et al. AIDS Res Hum Retroviruses. 1999 Jul 1;15(10):895-7. doi: 10.1089/088922299310601. AIDS Res Hum Retroviruses. 1999. PMID: 10408726 - Chemokine receptors and virus entry in the central nervous system.
Gabuzda D, Wang J. Gabuzda D, et al. J Neurovirol. 1999 Dec;5(6):643-58. doi: 10.3109/13550289909021293. J Neurovirol. 1999. PMID: 10602405 Review. - How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity.
Dimitrov DS. Dimitrov DS. Cell. 1997 Dec 12;91(6):721-30. doi: 10.1016/s0092-8674(00)80460-2. Cell. 1997. PMID: 9413981 Review. No abstract available.
Cited by
- A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization.
Wang W, Nie J, Prochnow C, Truong C, Jia Z, Wang S, Chen XS, Wang Y. Wang W, et al. Retrovirology. 2013 Feb 6;10:14. doi: 10.1186/1742-4690-10-14. Retrovirology. 2013. PMID: 23384254 Free PMC article. - Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism.
Yi Y, Isaacs SN, Williams DA, Frank I, Schols D, De Clercq E, Kolson DL, Collman RG. Yi Y, et al. J Virol. 1999 Sep;73(9):7117-25. doi: 10.1128/JVI.73.9.7117-7125.1999. J Virol. 1999. PMID: 10438797 Free PMC article. - The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses.
Bannert N, Schenten D, Craig S, Sodroski J. Bannert N, et al. J Virol. 2000 Dec;74(23):10984-93. doi: 10.1128/jvi.74.23.10984-10993.2000. J Virol. 2000. PMID: 11069993 Free PMC article. - Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages.
Worgall S, Connor R, Kaner RJ, Fenamore E, Sheridan K, Singh R, Crystal RG. Worgall S, et al. J Virol. 1999 Jul;73(7):5865-74. doi: 10.1128/JVI.73.7.5865-5874.1999. J Virol. 1999. PMID: 10364338 Free PMC article.
References
- Alkhatib G, Berger E A, Murphy P M, Pease J E. Determinants of HIV-1 coreceptor function on CC chemokine receptor 3: importance of both extracellular and transmembrane/cytoplasmic regions. J Biol Chem. 1997;272:20420–20426. - PubMed
- Alkhatib, G., and E. A. Berger. Unpublished data.
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources