ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases - PubMed (original) (raw)
ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases
T Koseki et al. Proc Natl Acad Sci U S A. 1998.
Abstract
We have identified and characterized ARC, apoptosis repressor with caspase recruitment domain (CARD). Sequence analysis revealed that ARC contains an N-terminal CARD fused to a C-terminal region rich in proline/glutamic acid residues. The CARD domain of ARC exhibited significant homology to the prodomains of apical caspases and the CARDs present in the cell death regulators Apaf-1 and RAIDD. Immunoprecipitation analysis revealed that ARC interacts with caspase-2, -8, and Caenorhabditis elegans CED-3, but not with caspase-1, -3, or -9. ARC inhibited apoptosis induced by caspase-8 and CED-3 but not that mediated by caspase-9. Further analysis showed that the enzymatic activity of caspase-8 was inhibited by ARC in 293T cells. Consistent with the inhibition of caspase-8, ARC attenuated apoptosis induced by FADD and TRADD and that triggered by stimulation of death receptors coupled to caspase-8, including CD95/Fas, tumor necrosis factor-R1, and TRAMP/DR3. Remarkably, the expression of human ARC was primarily restricted to skeletal muscle and cardiac tissue. Thus, ARC represents an inhibitor of apoptosis expressed in muscle that appears to selectively target caspases. Delivery of ARC by gene transfer or enhancement of its endogenous activity may provide a strategy for the treatment of diseases that are characterized by inappropriately increased cell death in muscle tissue.
Figures
Figure 1
Structure, sequence, and alignment of ARC with related proteins. (A) Schematic structure of human ARC. CARD and proline/glutamic acid-rich domains are shown as closed and open boxes. (B) The amino acid sequences of human and rat ARC are aligned. The identical residues in human and rat ARC were indicated by asterisk. (C) Alignment of the amino acid sequences of CARD domains of ARC, caspase-9, human caspase-2, RAIDD, and Apaf-1. The conserved residues in human and rat ARC were indicated by asterisk.
Figure 2
Expression of ARC in human tissues by Northern blot analysis. Poly(A)+ RNAs from various tissues were hybridized with a probe corresponding to the entire human ARC cDNA.
Figure 3
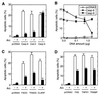
ARC is a negative regulator of apoptosis. 293T cells were transfected with pcDNA3, pcDNA3-ARC-Flag, and various expression plasmids as described in Material and Methods. Transfected cells were visualized with β-galactosidase substrate and scored for morphological feature of apoptosis. (A) Caspases were cotransfected with ARC (▪) or without ARC (□). In the experiment, 0.2 μg of plasmid DNA expressing caspase-4, -8, or -10 or 0.1 μg of Ced-3 or caspase-9 plasmid was used in the presence of 2 μg of pcDNA3-ARC-Flag or pcDNA3. (B) ARC inhibits caspase-induced apoptosis in a dose-dependent manner. 0.2 μg of plasmid expressing caspase-4, -8, -10, or pcDNA3 was used. The x axis represents amount of ARC plasmid DNA. Total amounts of plasmid DNA was 2.2 μg in all experiments presented in A and B. (C) ARC inhibits FADD, TRADD, and CLARP-induced apoptosis. The amount of plasmid DNA were: FADD (0.4 μg), TRADD (0.1 μg), CLARP (2.0 μg), or pcDNA (2.0 μg) in the presence of 2.0 μg of ARC or pcDNA3 plasmid. (D) ARC inhibits apoptosis induced by death receptors. The amount of plasmid DNA were: Fas (1.5 μg), TNFR1 (0.2 μg), TRAMP (1.0 μg), or pcDNA3 (2.0 μg) in the presence of 2.0 μg of ARC or pcDNA3 plasmid. Total amounts of plasmid DNA was 4.0 μg in all experiments presented in C and D.
Figure 4
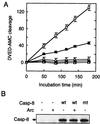
ARC suppresses the enzymatic activity of caspase-8 in intact cells. (A) 293T cells were cotransfected with 0.2 μg of pcDNA3-caspase-8-AU1 or pcDNA3-caspase-8-mut and 2 μg of pcDNA3-ARC-Flag or pcDNA3. Caspase-8 in cell extracts was immunoprecipitated with anti-AU1 antibody and immunoprecipitates were incubated with the fluorogenic substrate acetyl-Asp-Glu-Val-Asp7-amino-4-methylcoumarin. ○, pcDNA3 alone; •, pcDNA3-ARC-HA alone; □, pcDNA3-caspase-8-AU1 alone; ▪, pcDNA3-caspase-8-AU1 and pcDNA3-ARC-HA; ▵, pcDNA3-caspase-8-mt-AU1 alone. (B) AU1-tagged caspase-8 and caspase-8-mt were detected in immunoprecipitates with anti-AU1 by immunoblotting.
Figure 5
ARC interacts with caspase-2, -8, and Ced-3 but not with caspase-1, -3, and -9. (A) 293T cells were transfected with plasmids AU1-tagged caspase-2 or -8 and HA-tagged ARC. Lysates were immunoprecipitated with anti-AU1 antibody and immunoblotted with anti-HA antibody (Top). Total lysates (100 μg) were immunoblotted with anti-AU1 (Center) or anti-HA antibody (Bottom). (B and C) 293T cells were transfected with plasmids Flag-tagged caspase-1, -3, -9, or CED-3 and HA-tagged ARC. Lysates were immunoprecipitated with anti-Flag antibody and immunoblotted with anti-HA antibody (Top). Total lysates (100 μg) were immunoblotted with anti-Flag (Center) or anti-HA antibody (Bottom). (D) 293T cells were transfected with plasmids HA-tagged N-caspase-8 or C-caspase-8, and Flag-tagged ARC. Lysates were immunoprecipitated with anti-HA antibody and immunoblotted with anti-Flag antibody (Top). Total lysates (100 μg) were immunoblotted with anti-HA (Center) or anti-Flag antibody (Bottom).
Similar articles
- Casper is a FADD- and caspase-related inducer of apoptosis.
Shu HB, Halpin DR, Goeddel DV. Shu HB, et al. Immunity. 1997 Jun;6(6):751-63. doi: 10.1016/s1074-7613(00)80450-1. Immunity. 1997. PMID: 9208847 - Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95.
Chinnaiyan AM, O'Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM. Chinnaiyan AM, et al. Science. 1996 Nov 8;274(5289):990-2. doi: 10.1126/science.274.5289.990. Science. 1996. PMID: 8875942 - Caspases: the executioners of apoptosis.
Cohen GM. Cohen GM. Biochem J. 1997 Aug 15;326 ( Pt 1)(Pt 1):1-16. doi: 10.1042/bj3260001. Biochem J. 1997. PMID: 9337844 Free PMC article. Review. - Role of apoptosis repressor with caspase recruitment domain (ARC) in cell death and cardiovascular disease.
Zhang J, Zheng X, Wang P, Wang J, Ding W. Zhang J, et al. Apoptosis. 2021 Feb;26(1-2):24-37. doi: 10.1007/s10495-020-01653-x. Epub 2021 Feb 19. Apoptosis. 2021. PMID: 33604728 Review.
Cited by
- Crystal structure of caspase recruiting domain (CARD) of apoptosis repressor with CARD (ARC) and its implication in inhibition of apoptosis.
Jang TH, Kim SH, Jeong JH, Kim S, Kim YG, Park HH. Jang TH, et al. Sci Rep. 2015 Jun 3;5:9847. doi: 10.1038/srep09847. Sci Rep. 2015. PMID: 26038885 Free PMC article. - Specific acetylation of p53 by HDAC inhibition prevents DNA damage-induced apoptosis in neurons.
Brochier C, Dennis G, Rivieccio MA, McLaughlin K, Coppola G, Ratan RR, Langley B. Brochier C, et al. J Neurosci. 2013 May 15;33(20):8621-32. doi: 10.1523/JNEUROSCI.5214-12.2013. J Neurosci. 2013. PMID: 23678107 Free PMC article. - Caspase recruitment domain protein 6 is a microtubule-interacting protein that positively modulates NF-kappaB activation.
Dufner A, Pownall S, Mak TW. Dufner A, et al. Proc Natl Acad Sci U S A. 2006 Jan 24;103(4):988-93. doi: 10.1073/pnas.0510380103. Epub 2006 Jan 17. Proc Natl Acad Sci U S A. 2006. PMID: 16418290 Free PMC article. - ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening.
Xu T, Ding W, Ao X, Chu X, Wan Q, Wang Y, Xiao D, Yu W, Li M, Yu F, Wang J. Xu T, et al. Redox Biol. 2019 Jan;20:414-426. doi: 10.1016/j.redox.2018.10.023. Epub 2018 Nov 2. Redox Biol. 2019. PMID: 30415165 Free PMC article. - The PYRIN domain in signal transduction.
Stehlik C. Stehlik C. Curr Protein Pept Sci. 2007 Jun;8(3):293-310. doi: 10.2174/138920307780831857. Curr Protein Pept Sci. 2007. PMID: 17584123 Free PMC article. Review.
References
- Thompson C B. Science. 1995;267:1456–1462. - PubMed
- White E. Genes Dev. 1996;10:1–15. - PubMed
- Jacobson M D, Weil M, Raff M C. Cell. 1997;88:347–54. - PubMed
- Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Cell. 1996;87:171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous