Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression - PubMed (original) (raw)
Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression
Y E Whang et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The recently identified PTEN/MMAC1 gene is a candidate tumor suppressor implicated in multiple tumor types based on mutations or homozygous deletions of the gene in certain human cancers. No studies of PTEN/MMAC1 mRNA or protein expression in cancer cells have been reported, primarily because of significant numbers of normal cells contaminating most tumor samples and because of the lack of antibody reagents. We examined PTEN/MMAC1 in advanced prostate cancer for gene mutations or abnormalities in expression by using a series of recently derived xenografts free of normal human cells and a PTEN/MMAC1-specific antibody. Only 1 of 10 tumors contained a homozygous deletion of PTEN/MMAC1, and no mutations were detected in the entire coding region of the remaining nine xenografts. However, five of these showed reduced or absent PTEN/MMAC1 expression by Northern analysis and reverse transcription-PCR of mRNA. PTEN/MMAC1 mRNA expression was restored in nonexpressing prostate cancer cells by in vitro treatment with the demethylating agent 5-azadeoxycytidine. Alterations in PTEN/MMAC1 expression were confirmed at the protein level by immunoblot analysis, and immunohistochemical studies show that the endogenous wild-type PTEN/MMAC1 protein is localized exclusively in the cytoplasm. These results demonstrate that loss of PTEN/MMAC1 expression occurs frequently in advanced prostate cancer.
Figures
Figure 1
Homozygous deletion of PTEN/MMAC1. (A) Map of homozygous deletions. The approximate location of the PTEN/MMAC1 gene is shown as an open box. The ordered set of microsatellite markers on chromosome 10q23 are shown above the line. +, the presence of at least one allele as indicated by amplification of the correct size fragment by PCR of genomic DNA; −, the deletion of both alleles as indicated by the absence of the amplified fragment after PCR of genomic DNA. (B) Southern blot analysis. The filter containing _Hin_dIII-digested genomic DNA separated by electrophoresis was probed with the PTEN exon 5 probe. The band representing the pseudogene is indicated by ψ. The band representing the functional PTEN/MMAC1 gene is indicated by PTEN.
Figure 2
Northern blot analysis of PTEN/MMAC1 expression. (A) Total RNA from indicated cell lines and xenografts was analyzed by Northern blot analysis by using a 1.2-kb PTEN/MMAC1 probe. LAPC-4 AD and LAPC-4 AI represent the androgen-dependent and androgen-independent sublines, respectively. (B) The same Northern filter was stripped and reprobed for expression of glyceraldehyde-3-phosphate dehydrogenase.
Figure 3
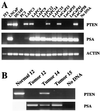
RT-PCR analysis of PTEN/MMAC1 expression. (A) Expression of PTEN/MMAC1, PSA, and human β-actin in indicated cell lines and xenografts was analyzed by RT-PCR, and ethidium bromide-stained agarose gels are shown. Because the PCR primers for β-actin are directed against the human sequence, the signal in murine NIH 3T3 cells is less pronounced than in other lanes. (B) Expression of PTEN/MMAC1 in peripheral blood leukocytes (normal 12) and PTEN/MMAC1 and PSA in primary prostate tumor tissues before establishment of xenografts was analyzed by RT-PCR. Tumors 12, 14, and 15 were used to establish LAPC-12, 14, and 15, respectively.
Figure 4
Restoration of PTEN/MMAC1 expression by 5-azadeoxycytidine. LuCaP-35 xenograft cells cultured in vitro were treated with 5-azadeoxycytidine at a concentration of 0, 1, or 2 μM, and cells were harvested for RNA after 4 days. Expression of PTEN/MMAC1 was detected by RT-PCR and was visualized by ethidium bromide staining. RT-PCR of PSA is shown as a control for the integrity of RNA in all samples.
Figure 5
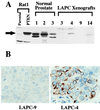
PTEN/MMAC1 protein expression. (A) Immunoblot of PTEN/MMAC1 protein. Rabbit polyclonal antibody raised against the C terminus of PTEN/MMAC1 protein was used to probe the whole cell extracts. Rat1-PTEN cells were infected with recombinant retrovirus expressing a cDNA for the PTEN/MMAC1 coding region. Cell extracts from normal prostate tissues from three different individuals (denoted as 1, 2, 3) are shown along with cell extracts from xenografts (LAPC-3, -4, -9, -14). The full length PTEN/MMAC1 protein is indicated by a solid arrow. (B) Immunohistochemical localization of PTEN/MMAC1 in LAPC-4 xenograft tumor (Right) reveals cytoplasmic staining with immunoperoxidase technique (brown). No staining is seen in LAPC-9 (Left) run in parallel on the same slide.
Similar articles
- Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues.
Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. Suzuki H, et al. Cancer Res. 1998 Jan 15;58(2):204-9. Cancer Res. 1998. PMID: 9443392 - Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas.
Rhei E, Kang L, Bogomolniy F, Federici MG, Borgen PI, Boyd J. Rhei E, et al. Cancer Res. 1997 Sep 1;57(17):3657-9. Cancer Res. 1997. PMID: 9288766 - PTEN/MMAC1 is infrequently mutated in pT2 and pT3 carcinomas of the prostate.
Dong JT, Sipe TW, Hyytinen ER, Li CL, Heise C, McClintock DE, Grant CD, Chung LW, Frierson HF Jr. Dong JT, et al. Oncogene. 1998 Oct 15;17(15):1979-82. doi: 10.1038/sj.onc.1202119. Oncogene. 1998. PMID: 9788441 - Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity.
Ali IU, Schriml LM, Dean M. Ali IU, et al. J Natl Cancer Inst. 1999 Nov 17;91(22):1922-32. doi: 10.1093/jnci/91.22.1922. J Natl Cancer Inst. 1999. PMID: 10564676 Review. - PTEN and myotubularin: novel phosphoinositide phosphatases.
Maehama T, Taylor GS, Dixon JE. Maehama T, et al. Annu Rev Biochem. 2001;70:247-79. doi: 10.1146/annurev.biochem.70.1.247. Annu Rev Biochem. 2001. PMID: 11395408 Review.
Cited by
- PTEN loss is associated with follicular variant of Middle Eastern papillary thyroid carcinoma.
Beg S, Siraj AK, Jehan Z, Prabakaran S, Al-Sobhi SS, Al-Dawish M, Al-Dayel F, Al-Kuraya KS. Beg S, et al. Br J Cancer. 2015 Jun 9;112(12):1938-43. doi: 10.1038/bjc.2015.169. Epub 2015 May 19. Br J Cancer. 2015. PMID: 25989274 Free PMC article. - Epigenetic regulation of prostate cancer.
Chin SP, Dickinson JL, Holloway AF. Chin SP, et al. Clin Epigenetics. 2011 Aug;2(2):151-69. doi: 10.1007/s13148-011-0041-7. Epub 2011 Jun 14. Clin Epigenetics. 2011. PMID: 22704335 Free PMC article. - Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer.
Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL. Mehrian-Shai R, et al. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5563-8. doi: 10.1073/pnas.0609139104. Epub 2007 Mar 19. Proc Natl Acad Sci U S A. 2007. PMID: 17372210 Free PMC article. - Male breast cancer in Cowden syndrome patients with germline PTEN mutations.
Fackenthal JD, Marsh DJ, Richardson AL, Cummings SA, Eng C, Robinson BG, Olopade OI. Fackenthal JD, et al. J Med Genet. 2001 Mar;38(3):159-64. doi: 10.1136/jmg.38.3.159. J Med Genet. 2001. PMID: 11238682 Free PMC article. - Detection of functional PTEN lipid phosphatase protein and enzyme activity in squamous cell carcinomas of the head and neck, despite loss of heterozygosity at this locus.
Snaddon J, Parkinson EK, Craft JA, Bartholomew C, Fulton R. Snaddon J, et al. Br J Cancer. 2001 Jun 15;84(12):1630-4. doi: 10.1054/bjoc.2001.1848. Br J Cancer. 2001. PMID: 11401316 Free PMC article.
References
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
- Steck P A, Pershouse M A, Jasser S A, Yung W K A, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
- Liaw D, Marsh D J, Li J, Dahia P L M, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. - PubMed
- Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin A F, Zeuthen J. Cancer Res. 1997;57:3660–3663. - PubMed
- Tashiro H, Blazes M S, Wu R, Cho K R, Bose S, Wang S I, Li J, Parsons R, Ellenson L H. Cancer Res. 1997;57:3935–3940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials