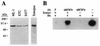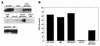Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7 - PubMed (original) (raw)
Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7
J M Sterner et al. Mol Cell Biol. 1998 May.
Abstract
A yeast two-hybrid screen was employed to identify human proteins that specifically bind the amino-terminal 400 amino acids of the retinoblastoma (Rb) protein. Two independent cDNAs resulting from this screen were found to encode the carboxy-terminal 137 amino acids of MCM7, a member of a family of proteins that comprise replication licensing factor. Full-length Rb and MCM7 form protein complexes in vitro, and the amino termini of two Rb-related proteins, p107 and p130, also bind MCM7. Protein complexes between Rb and MCM7 were also detected in anti-Rb immunoprecipitates prepared from human cells. The amino-termini of Rb and p130 strongly inhibited DNA replication in an MCM7-dependent fashion in a Xenopus in vitro DNA replication assay system. These data provide the first evidence that Rb and Rb-related proteins can directly regulate DNA replication and that components of licensing factor are targets of the products of tumor suppressor genes.
Figures
FIG. 1
Sequence of human MCM7 and characterization of MCM7 in human cells. (A) Amino acid sequence of human MCM7 (GenBank accession no. D55716). Underlined amino acids (denoted MCM7c) were encoded by two cDNAs identified in a yeast two-hybrid screen. An arrow indicates the amino-terminal end of the MCM7 protein (denoted MCM7n) encoded by a partial cDNA isolated by hybridization of a human cDNA library. (B) Immunoprecipitation of MCM7 proteins from human cells. ML-1 cells were metabolically labeled with [35S]methionine, nondenatured extracts were incubated with normal mouse serum (NMS) or mouse anti-MCM7c antiserum (αMCM7c), and precipitates were resolved on an 8% polyacrylamide gel. Molecular mass markers are indicated on the left. (C) Characterization of MCM7 proteins precipitated from human cells. As described above, ML-1 extracts were incubated with mouse MCM7c antiserum alone (−) or following preincubation of antiserum with an MBP-MCM7n fusion protein (MBP-MCM7n). The phosphorylation status of MCM7 proteins was assessed via incubation of αMCM7c precipitates with potato acid phosphatase (PAP).
FIG. 2
In vitro protein-binding assays. (A) Protein-binding assays with radiolabeled human Rb protein. Rabbit reticulocyte lysates programmed with human Rb cRNA were incubated with [35S]methionine, and the resulting radiolabeled proteins were divided evenly among three tubes. Aliquots were incubated with an anti-Rb monoclonal antibody (XZ77) or were incubated with equivalent amounts of GST-FSH15 or GST-MCM7n fusion proteins. Immunoprecipitates and bead-bound proteins were resolved on an 8% polyacrylamide gel. Molecular mass markers are indicated on the left. (B) Protein-binding assays with radiolabeled human MCM7n protein. Rabbit reticulocyte lysates programmed with human MCM7n cRNA were incubated with [35S]methionine, and equivalent amounts of radiolabeled proteins were incubated with rabbit anti-MCM7n antiserum (αMCM7n) or were incubated with GST-FSH15, GST-Rb, GST-Rb Δ89-140, GST-Rb Δ128-167, GST-Rb Δ249-309, GST-Rb Δ309-343, GST-Rb Δ343-389, GST-p107, and GST-p130 fusion proteins. Immunoprecipitates and bead-bound proteins were resolved on an 8% polyacrylamide gel. Molecular mass markers are indicated on the left.
FIG. 3
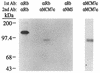
Association of Rb and MCM7 proteins in human cells. ML-1 cells were metabolically labeled with [35S]methionine, and nondenatured extracts were incubated with an anti-Rb monoclonal antibody (Ab [XZ77 or αRb]) or mouse anti-MCM7c antiserum (αMCM7c). The resulting immunoprecipitates were solubilized and reimmunoprecipitated with anti-Rb antibody (XZ77), mouse anti-MCM7c antiserum (αMCM7c), or normal mouse serum (αNMS), and precipitates were resolved on an 8% acrylamide gel. Molecular mass markers are indicated on the left.
FIG. 4
Association of human Rb and Xenopus MCM7 proteins in Xenopus egg extracts. (A) Detection of Xenopus MCM7 protein via Western blotting. Denatured cell extracts prepared from human ML-1 cells, two Xenopus cell lines (XTC and X477), and Xenopus eggs (Xenopus) were resolved on an 8% acrylamide gel, transferred to nitrocellulose, and probed with mouse anti-MCM7n antiserum. Molecular mass markers are indicated on the left. (B) Association of the amino-terminal 400 amino acids of human Rb protein with Xenopus MCM7. A histidine-tagged amino-terminal Rb protein harvested from baculovirus-infected cells was mixed with Xenopus egg extracts and incubated with preimmune (P) or immune (αMCM7n) rabbit anti-MCM7n antiserum. Histidine-tagged Rb protein in buffer (far right lane) and MCM7 immunoprecipitates were resolved on an 8% acrylamide gel, and Rb protein was detected by Western blotting with a monoclonal anti-Rb antibody (Ab) that binds an amino-terminal epitope (C36 [85]).
FIG. 5
Incubation of Rb protein with Xenopus egg extracts does not interfere with the formation of nuclei or cdk2 kinase activity. (A) Demembranated sperm chromatin was incubated in Xenopus interphase extracts (−) or extracts preincubated with an amino-terminal GST-Rb fusion protein (GST-Rb) or a control fusion protein (GST-FSH15). The formation of intact nuclei was assayed by staining with Hoechst 33258 and was visualized by fluorescence microscopy. Nuclei were somewhat variable in size, and representative examples are shown. (B) p13_suc_-Sepharose beads were incubated with Xenopus egg extracts (−) or extracts that had been precleared by incubation with Rb protein (GST-Rb). Bead-bound kinase activity was detected in an in vitro kinase assay using histone H1 as substrate. The position of histone H1 is indicated on the right.
FIG. 6
Inhibition of in vitro DNA replication by Rb family members. (A) Replication of sperm chromatin in Xenopus egg extracts treated with the indicated fusion proteins was established by pulse-labeling via incorporation of [α-32P]dCTP. For each extract, incorporation of [α-32P]dCTP into DNA was monitored following 60, 90, 120, and 150 min of incubation. (B) Quantification of DNA replication at the 90-min time point shown in panel A. Replication assays were quantified by scanning in a PhosphorImager.
References
- Blow J J, Laskey R A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–587. - PubMed
- Blow J J, Laskey R A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature (London) 1988;332:546–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources