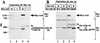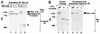Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos - PubMed (original) (raw)
Comparative Study
Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos
H Yamamoto et al. Mol Cell Biol. 1998 May.
Abstract
Using a yeast two-hybrid method, we identified a novel protein which interacts with glycogen synthase kinase 3beta (GSK-3beta). This protein had 44% amino acid identity with Axin, a negative regulator of the Wnt signaling pathway. We designated this protein Axil for Axin like. Like Axin, Axil ventralized Xenopus embryos and inhibited Xwnt8-induced Xenopus axis duplication. Axil was phosphorylated by GSK-3beta. Axil bound not only to GSK-3beta but also to beta-catenin, and the GSK-3beta-binding site of Axil was distinct from the beta-catenin-binding site. Furthermore, Axil enhanced GSK-3beta-dependent phosphorylation of beta-catenin. These results indicate that Axil negatively regulates the Wnt signaling pathway by mediating GSK-3beta-dependent phosphorylation of beta-catenin, thereby inhibiting axis formation.
Figures
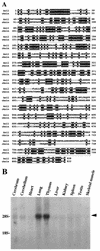
FIG. 1
Structure of Axil. (A) Amino acid sequence of Axil. Identical residues in Axil and rAxin are denoted by a black background. The RGS homologous and Dsh homologous domains are boxed. (B) Northern blot analysis of Axil. Total RNAs (20 μg/lane) of various rat tissues were probed with cDNA encoding Axil(1-148). The positions of 28S and 18S ribosomal RNAs are indicated. The arrowhead indicates the positions of Axil mRNA. The results shown are representative of two independent experiments.
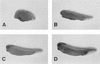
FIG. 2
Effect of Axil on axis formation of Xenopus embryos. (A and B) Dorsal injection of Axil mRNA. The embryo has no head region (DAI = 1) (A) or has microcephaly (DAI = 3) (B). (C) Ventral injection of Axil mRNA. (D) Control injection of Xglobin mRNA. Embryos were evaluated at the tail bud stage, and examples are shown.
FIG. 3
Interaction of Axil with GSK-3β. (A) Interaction of Axil with endogenous GSK-3β in COS cells. The lysates (20 μg of protein) of COS cells expressing Myc-Axil were probed with the anti-Myc and anti-GSK-3β antibodies (lane 1). The same lysates (500 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-Myc and anti-GSK-3β antibodies (lane 3). The lysates of COS cells transfected with empty vectors were used as controls (lanes 2 and 4). (B) Requirement of kinase activity of GSK-3β for its interaction with Axil. Myc-Axil was coexpressed with wild-type HA–GSK-3β (lanes 1 and 3) or HA–GSK-3βK85M (lanes 2 and 4) in COS cells, and the lysates were probed with the anti-Myc and anti-HA antibodies (lanes 1 and 2) or immunoprecipitated with the anti-HA antibody. The immunoprecipitates were then probed with the anti-Myc and anti-HA antibodies (lanes 3 and 4). IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin; WT, wild type HA–GSK-3β; 85M, HA–GSK-3βK85M. The arrows, small arrowhead, and large arrowhead indicate the positions of Myc-Axil, endogenous GSK-3β, and HA–GSK-3β or HA–GSK-3βK85M, respectively. The results shown are representative of three independent experiments.
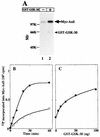
FIG. 4
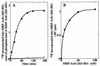
Phosphorylation of Axil by GSK-3β. (A) Autoradiography. Myc-Axil immunoprecipitated from COS cell lysates (250 μg of protein) was incubated with (lane 2) or without (lane 1) GST–GSK-3β (100 ng of protein) for 20 min, and the samples were subjected to SDS-PAGE followed by autoradiography. The arrow and arrowhead indicate the positions of Myc-Axil and GST–GSK-3β, respectively. (B) Time course. Myc-Axil immunoprecipitated from COS cell lysates was incubated with (•) or without (○) GST–GSK-3β (100 ng of protein) for the indicated periods of time. (C) Dose dependency. Myc-Axil was incubated with the indicated amounts of GST–GSK-3β for 20 min. The results shown are representative of four independent experiments.
FIG. 5
Interaction of Axil with β-catenin in intact cells (A) and in vitro (B). (A) The lysates (20 μg of protein) of COS cells expressing Myc-Axil were probed with the anti-Myc and anti-β-catenin antibodies (lane 1). The same lysates (500 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-Myc and anti-β-catenin antibodies (lane 3). The lysates of COS cells transfected with empty vectors were used as controls (lanes 2 and 4). (B) GST–β-catenin(full length) (lane 1), GST–N-terminal β-catenin (lane 2), and GST–C-terminal β-catenin (lane 3) (10 pmol each) were subjected to SDS-PAGE followed by Coomassie brilliant blue staining. After the lysates (500 μg of protein) of COS cells expressing Myc-Axil were incubated with GST–β-catenin (lane 4), GST–N-terminal β-catenin (lane 5), and GST–C-terminal β-catenin (lane 6) (50 pmol each), β-catenin and its deletion mutants were precipitated with glutathione Sepharose 4B. The precipitates were probed with the anti-Myc antibody. IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin; full, GST–β-catenin(full length); N, GST–N-terminal β-catenin; C, GST–C-terminal β-catenin. The arrows and arrowhead indicate the positions of Myc-Axil and endogenous β-catenin, respectively. The results shown are representative of three independent experiments.
FIG. 6
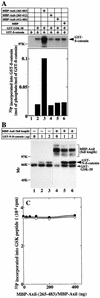
Complex formation of GSK-3β, Axil, and β-catenin. (A) Complex formation in intact cells. The lysates (20 μg of protein) of COS cells expressing HA–GSK-3β and Myc-Axil (lanes 1 and 3) and HA–GSK-3β alone (lanes 2 and 4) were probed with the anti-Myc, anti-β-catenin, and anti-HA antibodies (lanes 1 and 2). The same lysates (500 μg of protein) were immunoprecipitated with the anti-HA antibody, and the immunoprecipitates were probed with the anti-Myc, anti-β-catenin, and anti-HA antibodies (lanes 3 and 4). IP, immunoprecipitation, Ab, antibody; Ig, immunoglobulin. The arrow, large arrowhead, and small arrowhead indicate the positions of Myc-Axil, endogenous β-catenin, and HA–GSK-3β, respectively. (B) Deletion mutants of Axil. The hatched and empty boxes indicate the RGS and Dsh homologous domains, respectively. (C) Expression of Axil deletion mutants and their interaction with GSK-3β and β-catenin. The lysates (20 μg of protein) of COS cells expressing Myc-Axil(full length) (lane 1), Myc-Axil(1-670) (lane 2), Myc-Axil(682-838) (lane 3), Myc-Axil(1-265) (lane 4), and Myc-Axil(265-483) (lane 5) were probed with the anti-Myc antibody (left panel). The same lysates (500 to 1,000 μg of protein) (lanes 6 to 10) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-GSK-3β and anti-β-catenin antibodies (right panel). The small and large arrowheads indicate the positions of endogenous GSK-3β and β-catenin, respectively. (D) Different binding sites of Axil for GSK-3β and β-catenin. After GST–GSK-3β (lanes 1 to 4) and GST–β-catenin (lanes 5 to 8) (8 pmol each) were incubated with MBP-Axil(265-483) (lanes 1 and 5), MBP-Axil(265-412) (lanes 2 and 6), MBP-Axil(412-483) (lanes 3 and 7), or MBP (lanes 4 and 8) (2 pmol each) immobilized on amylose resin, MBPs fused to proteins were precipitated by centrifugation. The precipitates were probed with the anti-GSK-3β and anti-β-catenin antibodies. The small and large arrowheads indicate the positions of GST–GSK-3β and GST–β-catenin, respectively. (E) Phosphorylation of Axil(265-483) by GSK-3β. MBP-Axil(265-483) (200 ng of protein) was incubated with (lane 2) or without (lane 1) GST–GSK-3β (100 ng of protein) for 30 min. The arrow indicates the position of MBP-Axil(265-483). The results shown are representative of three independent experiments.
FIG. 7
Kinetics for the phosphorylation of Axil(265-483) by GSK-3β. (A) Time course. MBP-Axil(265-483) (200 ng of protein) purified from E. coli was incubated with (•) or without (○) GST–GSK-3β (100 ng of protein) for the indicated periods of time. (B) Dose dependency. The indicated concentrations of MBP-Axil(265-483) were incubated with GST–GSK-3β (100 ng of protein) for 20 min. The results shown are representative of three independent experiments.
FIG. 8
Phosphorylation of β-catenin by GSK-3β in the presence of Axil. (A) Effect of MBP-Axil(265-483) on GSK-3β-dependent phosphorylation of β-catenin. GST–β-catenin (2 μg of protein) was incubated with GST–GSK-3β (600 ng of protein) in the presence of MBP-Axil(265-483) (lane 3), MBP-Axil(265-412) (lane 4), MBP-Axil(412-483) (lane 5), or MBP (lane 6) (200 ng of protein each) for 30 min. As a control, GST–β-catenin was incubated with (lane 2) or without (lane 1) GST–GSK-3β. (Upper panel) Autoradiography is shown. (Lower panel) The radioactivities incorporated into GST–β-catenin were counted and the stoichiometry of the phosphorylation was calculated. (B) Effect of full-length Axil on GSK-3β-dependent phosphorylation of β-catenin. The indicated amounts of GST–N-terminal β-catenin were incubated with GST–GSK-3β (400 ng of protein) in the presence (lanes 4 to 6) and absence (lanes 1 to 3) of MBP-Axil (160 ng of protein). GST–N-β-catenin, GST–N-terminal β-catenin. (C) Effect of Axil on GSK-3β activity. GST–GSK-3β (400 ng of protein) was incubated with 50 μM GSK peptide 1 in the presence of the indicated amounts of MBP-Axil(265-483) (•) or MBP-Axil (○). The results shown are representative of five independent experiments.
Similar articles
- Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin.
Hedgepeth CM, Deardorff MA, Rankin K, Klein PS. Hedgepeth CM, et al. Mol Cell Biol. 1999 Oct;19(10):7147-57. doi: 10.1128/MCB.19.10.7147. Mol Cell Biol. 1999. PMID: 10490650 Free PMC article. - Modulation of Wnt signaling by Axin and Axil.
Kikuchi A. Kikuchi A. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review. - Inhibition of Wnt signaling pathway by a novel axin-binding protein.
Kadoya T, Kishida S, Fukui A, Hinoi T, Michiue T, Asashima M, Kikuchi A. Kadoya T, et al. J Biol Chem. 2000 Nov 24;275(47):37030-7. doi: 10.1074/jbc.M005984200. J Biol Chem. 2000. PMID: 10944533 - Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Ikeda S, et al. EMBO J. 1998 Mar 2;17(5):1371-84. doi: 10.1093/emboj/17.5.1371. EMBO J. 1998. PMID: 9482734 Free PMC article. - New steps in the Wnt/beta-catenin signal transduction pathway.
Sakanaka C, Sun TQ, Williams LT. Sakanaka C, et al. Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
- Coordinated changes in the expression of Wnt pathway genes following human and rat peripheral nerve injury.
van Vliet AC, Lee J, van der Poel M, Mason MRJ, Noordermeer JN, Fradkin LG, Tannemaat MR, Malessy MJA, Verhaagen J, De Winter F. van Vliet AC, et al. PLoS One. 2021 Apr 13;16(4):e0249748. doi: 10.1371/journal.pone.0249748. eCollection 2021. PLoS One. 2021. PMID: 33848304 Free PMC article. - Barx2 and Pax7 Regulate Axin2 Expression in Myoblasts by Interaction with β-Catenin and Chromatin Remodelling.
Hulin JA, Nguyen TD, Cui S, Marri S, Yu RT, Downes M, Evans RM, Makarenkova H, Meech R. Hulin JA, et al. Stem Cells. 2016 Aug;34(8):2169-82. doi: 10.1002/stem.2396. Epub 2016 Jun 6. Stem Cells. 2016. PMID: 27144473 Free PMC article. - Impaired neural development caused by inducible expression of Axin in transgenic mice.
Yu HM, Liu B, Costantini F, Hsu W. Yu HM, et al. Mech Dev. 2007 Feb;124(2):146-56. doi: 10.1016/j.mod.2006.10.002. Epub 2006 Oct 11. Mech Dev. 2007. PMID: 17123792 Free PMC article. - Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription.
Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Smalley MJ, et al. EMBO J. 1999 May 17;18(10):2823-35. doi: 10.1093/emboj/18.10.2823. EMBO J. 1999. PMID: 10329628 Free PMC article. - Catalytic roles of yeast GSK3beta/shaggy homolog Rim11p in meiotic activation.
Malathi K, Xiao Y, Mitchell AP. Malathi K, et al. Genetics. 1999 Nov;153(3):1145-52. doi: 10.1093/genetics/153.3.1145. Genetics. 1999. PMID: 10545448 Free PMC article.
References
- Behrens J, von Kries J P, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Bhanot P, Brink M, Samos C H, Hsieh J C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. - PubMed
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
- Cadigan K M, Nusse R. Wnt Meeting 1996. Biochim Biophys Acta. 1997;1332:R1–R5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases