In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules - PubMed (original) (raw)
In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules
C E Futter et al. J Cell Biol. 1998.
Abstract
Human transferrin receptors (TR) and receptors for polymeric immunoglobulins (pIgR) expressed in polarized MDCK cells maintain steady-state, asymmetric distributions on the separate basolateral and apical surfaces even though they are trafficking continuously into and across these cells. The intracellular mechanisms required to maintain these asymmetric distributions have not been located. Here we show that TR and pIgR internalize from both surfaces to a common interconnected endosome compartment that includes tubules with buds coated with clathrin lattices. These buds generate vesicles that carry TR to the basolateral border. The lattices contain gamma-adaptin and are dispersed by treatment with brefeldin A (BFA). Since BFA treatment abrogates the vectorial trafficking of TR in polarized MDCK cells, we propose that the clathrin-coated domains of the endosome tubules contain the polarized sorting mechanism responsible for their preferential basolateral distribution.
Figures
Figure 1
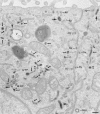
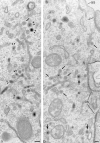
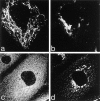
Codistribution of TF and IgA tracers applied from basolateral and apical surfaces of polarized cells. Polarized MDCK expressing TR and pIgR were incubated basolaterally with TF-HRP (60 min at 37°C) and apically with IgA-gold (30 min at 37°C). Endosomal elements labeled with both tracers are distributed throughout the cytoplasm and include 0.3-μm-diam vacuoles (V; with a tubular extension, arrowhead), narrower branching tubules (small arrows), larger 100– 150-nm-diam tubules (large arrows) and 60-nm-diam basolateral vesicles (bv). Basolaterally applied TF-HRP is not present on the apical membrane, and the coated pits and vesicles derived from this membrane (ap) are identifiable because they have only been reached by apical tracer. bcp indicates a coated vesicle derived from basolateral surface containing only TF-HRP and has, typically, a peripheral distribution of DAB reaction product. Double arrowheads indicate coated buds on 60-nm tubules. L, lysosome. Bar, 0.1 μm.
Figure 2
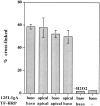
Cross-linking of TF and IgA tracers internalized from the same and from opposite sides of the monolayer at low temperature. Polarized MDCK cells expressing TR and pIgR were incubated with radio-labeled or HRP-conjugated tracers from either the same or opposite sides of the monolayer, as indicated, for 2 h at 20°C. DAB cross-linking was then performed, as described in Materials and Methods. Results are means ± SD of three or four observations.
Figure 3
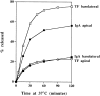
Polarized sorting of TF and IgA at 37°C. Polarized MDCK cells expressing TR and pIgR were preloaded basolaterally for 2 h at 20°C with 125I-IgA (filled symbols) or 125I-TF (open symbols) and chased at 37°C, and apical (circles) and basolateral (squares) media were collected. Results are mean ± SD of three to six observations. SD are smaller than the symbols.
Figure 4
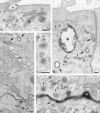
Penetration of gold-labeled TR from the apical surface into transcytotic elements preloaded with TF-HRP from the basolateral surface. (a) At the apical border, apically applied gold-labeled TR are present in coated pits (acp) and have gained access to endosomal elements (small arrowheads) containing TF-HRP that has been loaded from the basolateral surface. (b) At the basolateral border, TF-HRP is distributed in the basolateral space and in coated pits/vesicles (bcp). Deeper in the cell, the TF-HRP is present in tubules and vacuoles of variable diameter (arrows) and in small (60-nm-diam) basolateral vesicles (small arrow) that also contain apically derived gold-labeled TR. (c) Two 60-nm-diam vesicles (one coated, double arrows) showing (1) the homogeneous distribution of DAB reaction product due to TF-HRP derived from the basolateral border and (2) the central cluster of gold-labeled TR (internalized from the apical surface) that characterize the content of basolateral vesicles. (d) Endosomal vacuole (*) surrounded by tubules and vesicles containing apically and basolaterally derived tracer. Large arrows indicate larger tubules with a peripheral distribution of DAB reaction product similar to the vacuole. Small arrows indicate 60-nm-diam tubules and vesicles filled with DAB reaction product. In cross-sections of these narrow tubules, the gold particles occupy a central position, which in longitudinal sections are often seen in single file. Double arrows indicate a clathrin-coated bud. (e) Basolateral border (BL) showing 60-nm-diam basolateral tubules/vesicles (arrows) containing apically derived gold TR tracer and a smooth surfaced invagination (small asterisk) where an exocytotic vesicle has fused. The density of the DAB reaction product within the exocytotic invagination is similar to that given by the TF-HRP in the lateral space (and much stronger than in the unfused vesicles in the peripheral cytoplasm), suggesting that there is a rapid equilibration of intra- and extracellular tracer when vesicles fuse. The large asterisk indicates an endocytotic invagination identifiable because it is larger (∼100-nm-diam) and is clathrin-coated. Bars, 0.1 μm.
Figure 5
Coated buds and 60-nm vesicles in polarized cells containing basolaterally directed tracers. Endosomal tubules containing basolaterally applied TF-HRP and apically applied IgA-gold. The TF-HRP serves to identify endosomal elements accessible from the basolateral surface, and the IgA-gold demonstrates that these elements also process apically derived tracer. Fig. 3 shows that TR are routed preferentially to the basolateral border, and significant amounts of the IgA are also routed basolaterally from the endosome. (a) Double arrowheads indicate coated buds containing concentrated TF-HRP reaction product. The small arrow indicates clathrin lattice. (b) Cytoplasmic coats are seen on the tubules (small arrows) and on apparently free 60-nm-diam vesicles (larger arrows) that contain concentrated TF-HRP and IgA-gold tracer. as, IgA-gold on apical surface; J, junctional complex; bs, basolateral surface with TF-HRP within intercellular space. Bars, 0.1 μm.
Figure 6
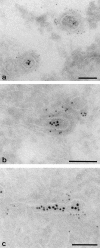
Labeling cytoplasmic domains of TR and coat proteins on 60-nm-diam tubules and vesicles loaded basolaterally with TF-HRP in polarized monolayers permeabilized with digitonin. (a) Thick section showing distribution of H68.4-labeled TR in a 100-nm-diam form of tubule that extends into a 60-nm-diam form with a terminal bud. Permeabilized cell treated with Tris and Triton X-100 to remove clathrin lattices. TF-HRP reaction product and the gold labeling (arrowheads) indicates some concentration as the tubule narrows. (b) 60-nm-diam vesicle containing internalized TF-HRP. The cytoplasmic domains of the TR in this free vesicle are labeled with 5-nm H68.4 antibody. Arrow indicates the plasma membrane. (c) Thin section showing distribution of H68.4–gold on TF-HRP–containing endosomal elements (asterisks) and basolateral vesicles. One basolateral vesicle is coated and unlabeled with gold (small arrow) and the other is labeled (arrowheads). Large arrows indicate basolateral plasma membrane. (d) Thick section showing 60-nm-diam endosomal tubules labeled for clathrin (small arrows). (e) Thick section of 60-nm-diam endosomal tubules labeled for γ-adaptin with 5-nm-gold (small arrows). The tubules contain apically derived TR gold tracer in linear array (arrowheads) and the coated bud contains a central cluster of six particles. Bars, 0.05 μm.
Figure 7
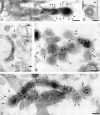
Clathrin and γ-adaptin on endosomal tubules in cells grown on solid substrata. Cells grown on solid substrata loaded with TF-HRP and IgA-gold (12 nm), prepared for cryosections and (a) clathrin (Pansy) and (b and c) γ-adaptin localized with immunogold. The endocytosed 12-nm gold tracer identifies the tubules as endosomal, and the 5-nm particles show that the coats contain clathrin and γ-adaptin. Bars, 0.1 μm.
Figure 8
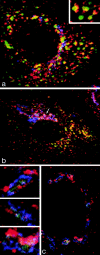
Immunofluorescent localization of γ-adaptin in cells grown on solid substrata. (a and b) MDCK cells stably expressing ST-HRP were transiently transfected with human TR and were incubated for 1 h at 37°C with TF-FITC. They were triple labeled for γ-adaptin (red), TF-FITC (green), and ST-HRP (blue). (a) In the peripheral cytoplasm, γ-adaptin is closely associated with endocytosed TF-FITC. In the enlarged structures shown in the inset, γ-adaptin appears to cap the tubules extending from the TF-FITC–containing vacuoles. (b) In the Golgi region, the γ-adaptin is closely associated with ST-HRP (arrow), but overlap between TF-FITC and the ST-HRP is negligible. (c) Triple label for γ-adaptin (red), ST-HRP (green), and β′-COP (blue). In addition to labeling the punctate structures in the peripheral cytoplasm, γ-adaptin is closely associated with β′-COP label in the ST-HRP– containing Golgi elements. Seen at high magnification (insets), it is clear that the Golgi elements contain discrete domains in which either γ-adaptin or COP1 are concentrated.
Figure 9
The effects of BFA on the Golgi, endosomes, and their associated coat proteins. Cells expressing ST-HRP and transiently transfected with human TR were incubated with TF-FITC for 1 h at 37°C and then treated with 5 μg/ml BFA for 10 min at 37°C. (a) TF-FITC–containing endosomes show dramatic tubulation while (b) ST-HRP–containing _trans_-Golgi elements develop relatively few tubules. (c) BFA causes γ-adaptin from both Golgi and peripheral locations to become dispersed while (d) β′-COP remains concentrated in the Golgi area.
Figure 10
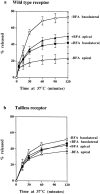
The effects of BFA on polarized sorting of TF. Polarized monolayers preloaded basolaterally at 37°C for 2 h with 125I-TF were stripped of surface label and then incubated at 37°C with (filled symbols) or without (open symbols) BFA, and apical (triangles) and basolateral (squares) media were collected. (a) In cells expressing wild-type TR, TF is preferentially released to the basolateral surface, but in the presence of BFA, release to apical and basolateral surfaces is approximately equal. (b) In cells expressing tailless TR, TF release to the separate surfaces is approximately the same with and without BFA treatment. All results are mean ± SD of three to six observations.
Similar articles
- Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells.
Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Gibson A, et al. J Cell Biol. 1998 Oct 5;143(1):81-94. doi: 10.1083/jcb.143.1.81. J Cell Biol. 1998. PMID: 9763422 Free PMC article. - Brefeldin A rapidly disrupts plasma membrane polarity by blocking polar sorting in common endosomes of MDCK cells.
Wang E, Pennington JG, Goldenring JR, Hunziker W, Dunn KW. Wang E, et al. J Cell Sci. 2001 Sep;114(Pt 18):3309-21. doi: 10.1242/jcs.114.18.3309. J Cell Sci. 2001. PMID: 11591819 - Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism.
Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Odorizzi G, et al. J Cell Biol. 1996 Oct;135(1):139-52. doi: 10.1083/jcb.135.1.139. J Cell Biol. 1996. PMID: 8858169 Free PMC article. - Trafficking of lysosomal membrane proteins in polarized kidney cells.
Hunziker W, Simmen T, Höning S. Hunziker W, et al. Nephrologie. 1996;17(7):347-50. Nephrologie. 1996. PMID: 8987042 Review. - Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes.
Gonzalez A, Rodriguez-Boulan E. Gonzalez A, et al. FEBS Lett. 2009 Dec 3;583(23):3784-95. doi: 10.1016/j.febslet.2009.10.050. Epub 2009 Oct 23. FEBS Lett. 2009. PMID: 19854182 Free PMC article. Review.
Cited by
- Receptor complexes cotransported via polarized endocytic pathways form clusters with distinct organizations.
Wallrabe H, Bonamy G, Periasamy A, Barroso M. Wallrabe H, et al. Mol Biol Cell. 2007 Jun;18(6):2226-43. doi: 10.1091/mbc.e06-08-0700. Epub 2007 Apr 4. Mol Biol Cell. 2007. PMID: 17409357 Free PMC article. - Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells.
Waguri S, Dewitte F, Le Borgne R, Rouillé Y, Uchiyama Y, Dubremetz JF, Hoflack B. Waguri S, et al. Mol Biol Cell. 2003 Jan;14(1):142-55. doi: 10.1091/mbc.e02-06-0338. Mol Biol Cell. 2003. PMID: 12529433 Free PMC article. - Gyrating clathrin: highly dynamic clathrin structures involved in rapid receptor recycling.
Zhao Y, Keen JH. Zhao Y, et al. Traffic. 2008 Dec;9(12):2253-64. doi: 10.1111/j.1600-0854.2008.00819.x. Epub 2008 Sep 13. Traffic. 2008. PMID: 18817526 Free PMC article. - A novel AAK1 splice variant functions at multiple steps of the endocytic pathway.
Henderson DM, Conner SD. Henderson DM, et al. Mol Biol Cell. 2007 Jul;18(7):2698-706. doi: 10.1091/mbc.e06-09-0831. Epub 2007 May 9. Mol Biol Cell. 2007. PMID: 17494869 Free PMC article. - Involvement of the AP-1 adaptor complex in early steps of phagocytosis and macropinocytosis.
Lefkir Y, Malbouyres M, Gotthardt D, Ozinsky A, Cornillon S, Bruckert F, Aderem AA, Soldati T, Cosson P, Letourneur F. Lefkir Y, et al. Mol Biol Cell. 2004 Feb;15(2):861-9. doi: 10.1091/mbc.e03-06-0365. Epub 2003 Nov 14. Mol Biol Cell. 2004. PMID: 14617812 Free PMC article.
References
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996;6:315–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous