Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia - PubMed (original) (raw)
Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia
S Namura et al. J Neurosci. 1998.
Abstract
We examined the expression, activation, and cellular localization of caspase-3 (CPP32) using immunohistochemistry, immunoblots, and cleavage of the fluorogenic substrate N-benzyloxycarbonyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (zDEVD-afc) in adult mouse brain after temporary (2 hr) middle cerebral artery occlusion produced by filament insertion into the carotid artery. Immunoreactive caspase-3p32 but not its cleavage product caspase-3p20 was constitutively expressed in neurons throughout brain and was most prominent in neuronal perikarya within piriform cortex. Caspase-like enzyme activity was elevated in brain homogenate 0-3 hr after reperfusion and reached a peak within 30 to 60 min. Caspase-3p20 immunoreactivity became prominent in neuronal perikarya within the middle cerebral artery territory at the time of reperfusion and on immunoblots 1-12 hr later. DNA laddering (agarose gels) and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL)-stained cells were detected 6-24 hr after reperfusion. At 12-24 hr, immunoreactive p20 was visualized in TUNEL-positive cells, a finding also observed in apoptotic mouse cerebellar granule cells on postnatal day 5. Together, these observations suggest the existence of a time-dependent evolution of ischemic injury characterized by the close correspondence between caspase-like enzyme activation and an associated increase in immunoreactive product (caspase-3p20) beginning at or before reperfusion and followed several hours later by morphological and biochemical features of apoptosis.
Figures
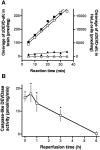
Fig. 1.
A, Cleavage of the caspase fluorogenic substrate zDEVD-afc (12.5 μ
m
) in homogenates of HeLa cells and brain tissue homogenates. Reaction was monitored every 5 min for 35 min by spectrofluorometry in apoptotic HeLa cell lysate, which was isolated after treatment with 1 μ
m
staurosporine for 3 hr (□) or in control cell lysate (▵). Caspase-like DEVDase activity was also measured in brain tissue homogenates from the ischemic (▪) or contralateral (▴) hemisphere 1 hr after reperfusion after 2 hr of middle cerebral artery occlusion. The data are from a single set of representative experiments that were highly reproducible. B, Caspase-like enzyme activity increases in brain homogenates after reperfusion after 2 hr occlusion of left middle cerebral artery. Enzyme activity measured as described above is expressed as picomole of substrate cleaved per milligram of protein per minute. Data show mean ± SEM of four to eight experiments assayed in duplicate. *p < 0.05 indicates a significant difference compared with sham-operated animals.
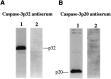
Fig. 2.
The specificity of antisera detecting either caspase-3p32 (A) or caspase-3p20 (B) is shown by immunoblots of lysates from mouse cerebellum on postnatal day 5 (P5). Ten micrograms of protein were loaded per lane, and the membrane was probed with 1:1500 dilution.A, A band corresponding to caspase-3p32 was detected (lane 1) that disappeared after preadsorption with peptide immunogen (lane 2). B, Immunoblots detected caspase-3p20 in lysates from mouse cerebellum (P5) (lane 1) but did not detect caspase-3p32. The p20 band disappeared after preadsorption with peptide immunogen (lane 2).
Fig. 3.
Time-dependent changes in caspase-3 during reperfusion after 2 hr middle cerebral artery occlusion. Brain lysates (10 μg/lane) were subjected to SDS-PAGE and immunoblot analysis using either caspase-3p32 (A) or caspase-3p20 (B) antiserum as described in Figure 2. Caspase-3p32 band was present in normal brain and did not change over time when measured in four different experiments (see Materials and Methods). Cleavage product (p20) increased 1–6 hr after reperfusion in the left (L, ischemic) compared with right (R, contralateral) and normal brain (N). A faint p20 band is also present at 12 hr. α-tubulin was used as an internal control (bottom). The experiment was repeated four times; a representative experiment is shown.
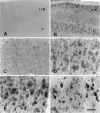
Fig. 4.
Immunohistochemical staining of normal (A–D) and ischemic (E) brains using caspase-3p32 or caspase-3p20 antiserum. Caspase-3p32 immunoreactivity (A, B) was detected in neuronal perikarya (large arrows) and dendrites (small arrows), in 40 μm sections from piriform (A) or in parietal cortex (B) counterstained with thionine hydrochloride. Frequently, cells in the piriform cortex contained intense caspase-3p32 immunoreactivity (A, arrowhead). Preadsorption with peptide immunogen (see Materials and Methods) markedly blocked the staining in piriform cortex (C). By contrast, normal brain stained poorly with anti-caspase-3p20 (piriform cortex) (D). Within the penumbra of ischemic cortex, caspase-3p32 staining was prominent in neuronal cytoplasm 24 hr after reperfusion (E). Scale bar, 15 μm.
Fig. 5.
Caspase-3p20 immunohistochemical staining of tissue sections (40 μm) from ischemic mouse parietal cortex after 2 hr middle cerebral artery occlusion and reperfusion. High-power photomicrographs (C–F) were taken from lamina V. Caspase-3p20 immunoreactivity was detected as early as 5 min after reperfusion in ischemic cortex (B, D) compared with the contralateral side (A, C) and was found within cytoplasm of cortical neurons after 5 min (B, D), 1 hr (E), and 24 hr (F) of reperfusion. Caspase-3p20 staining did not change in the contralateral hemisphere at these times (not shown). At 1 and 24 hr, coarse granular immunoproducts were densely concentrated in neurons (E, F). II, III, and V refer to cortical laminae (n = 3 animals per time point). Scale bar (shown in F): A, B, 120 μm; C–F, 15 μm.
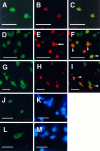
Fig. 6.
Confocal microscopic images document the localization of caspase-3p20 immunoreactivity and TUNEL in single tissue sections (40 μm) from normal cerebellar hemisphere (postnatal day 5, P5) (A–C) and ischemic striatum (D–F) and parietal cortex (G–I) after 2 hr middle cerebral artery occlusion and 12 hr (D–F) or 24 hr (G–I) reperfusion. Immunoreactivity and TUNEL were visualized with Bodipy fluorescein (A, D, G:green) and Cy3 (B, E, H:red), respectively. Almost every caspase-3p20 immunoreactive cerebellar granule cell at P5 was TUNEL positive. Two representative cells are shown here (C). Many ischemic cells contained both immunoreactive staining (p20) and TUNEL (F, I, arrowheads). Note a typical apoptotic body in a TUNEL-positive striatal cell (E, arrow). After 12 hr of reperfusion caspase-3p20 staining looked as if it was localized within the nucleus of some striatal cells [immunofluorescence staining (J) combined with Hoechst 33342 staining (K) using conventional fluorescence microscopy]. Within cortical cells, however, the pattern of p20 differed at 24 hr (L, p20 immunostaining; M, Hoechst 33342 staining), which may reflect the more rapid rate of ischemic evolution in striatum. Scale bar, 10 μm.
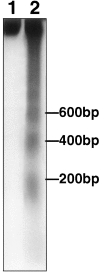
Fig. 7.
DNA laddering was detected by agarose gel electrophoresis in ipsilateral (lane 2) but not contralateral striatum (lane 1) 12 hr after reperfusion after 2 hr middle cerebral artery occlusion. Laddering appeared as early as 6 hr and was sustained at 24 hr as well (see Results). DNA was end-labeled with [32P]ddATP, electrophoresed on a 2% agarose gel, and autoradiographed. A ladder corresponding to 200, 400, and 600 bp is shown.
Similar articles
- Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia.
Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Chen J, et al. J Neurosci. 1998 Jul 1;18(13):4914-28. doi: 10.1523/JNEUROSCI.18-13-04914.1998. J Neurosci. 1998. PMID: 9634557 Free PMC article. - Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family.
Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gómez-Isla T, Hyman BT, Moskowitz MA. Endres M, et al. J Cereb Blood Flow Metab. 1998 Mar;18(3):238-47. doi: 10.1097/00004647-199803000-00002. J Cereb Blood Flow Metab. 1998. PMID: 9498840 - Inhibition of caspase-3 activation and apoptosis is involved in 3-nitropropionic acid-induced ischemic tolerance to transient focal cerebral ischemia in rats.
Zhu HC, Gao XQ, Xing Y, Sun SG, Li HG, Wang YF. Zhu HC, et al. J Mol Neurosci. 2004;24(2):299-305. doi: 10.1385/JMN:24:2:299. J Mol Neurosci. 2004. PMID: 15456943 - Prolonged therapeutic window for ischemic brain damage caused by delayed caspase activation.
Fink K, Zhu J, Namura S, Shimizu-Sasamata M, Endres M, Ma J, Dalkara T, Yuan J, Moskowitz MA. Fink K, et al. J Cereb Blood Flow Metab. 1998 Oct;18(10):1071-6. doi: 10.1097/00004647-199810000-00003. J Cereb Blood Flow Metab. 1998. PMID: 9778183 - Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice.
Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Shibata M, et al. Neuroscience. 2003;118(2):491-9. doi: 10.1016/s0306-4522(02)00910-7. Neuroscience. 2003. PMID: 12699784
Cited by
- Paeonol Protects Memory after Ischemic Stroke via Inhibiting β-Secretase and Apoptosis.
Su SY, Cheng CY, Tsai TH, Hsieh CL. Su SY, et al. Evid Based Complement Alternat Med. 2012;2012:932823. doi: 10.1155/2012/932823. Epub 2012 Mar 13. Evid Based Complement Alternat Med. 2012. PMID: 22474531 Free PMC article. - Protective Effect of Mangifera indica Linn., Cocos nucifera Linn., and Averrhoa carambola Linn. Extracts against Ultraviolet B-Induced Damage in Human Keratinocytes.
Ronpirin C, Pattarachotanant N, Tencomnao T. Ronpirin C, et al. Evid Based Complement Alternat Med. 2016;2016:1684794. doi: 10.1155/2016/1684794. Epub 2016 Mar 9. Evid Based Complement Alternat Med. 2016. PMID: 27057195 Free PMC article. - Time course, distribution and cell types of induction of transforming growth factor betas following middle cerebral artery occlusion in the rat brain.
Pál G, Vincze C, Renner É, Wappler EA, Nagy Z, Lovas G, Dobolyi A. Pál G, et al. PLoS One. 2012;7(10):e46731. doi: 10.1371/journal.pone.0046731. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056426 Free PMC article. - Brain endothelial cell death: modes, signaling pathways, and relevance to neural development, homeostasis, and disease.
Rizzo MT, Leaver HA. Rizzo MT, et al. Mol Neurobiol. 2010 Aug;42(1):52-63. doi: 10.1007/s12035-010-8132-6. Epub 2010 Apr 21. Mol Neurobiol. 2010. PMID: 20407845 Review. - Cell death in trichomonads: new insights.
Mariante RM, Vancini RG, Benchimol M. Mariante RM, et al. Histochem Cell Biol. 2006 May;125(5):545-56. doi: 10.1007/s00418-005-0098-5. Epub 2005 Nov 5. Histochem Cell Biol. 2006. PMID: 16273383
References
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
- Asahi M, Hoshimaru M, Uemura Y, Tokime T, Kojima M, Ohtsuka T, Matsuura N, Aoki T, Shibahara K, Kikuchi H. Expression of interleukin-1β converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17:11–18. - PubMed
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
- Bertrand R, Solary E, O’Connor P, Kohn KW, Pommier Y. Induction of common pathway of apoptosis by staurosporine. Exp Cell Res. 1994;211:314–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials