Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells - PubMed (original) (raw)
. 1998 May;9(5):993-1006.
doi: 10.1091/mbc.9.5.993.
Affiliations
- PMID: 9571235
- PMCID: PMC25324
- DOI: 10.1091/mbc.9.5.993
Free PMC article
Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells
S Yamashiro et al. Mol Biol Cell. 1998 May.
Free PMC article
Abstract
Fascin is an actin-bundling protein that is found in membrane ruffles, microspikes, and stress fibers. The expression of fascin is greatly increased in many transformed cells, as well as in specialized normal cells including neuronal cells and antigen-presenting dendritic cells. A morphological characteristic common to these cells expressing high levels of fascin is the development of many membrane protrusions in which fascin is predominantly present. To examine whether fascin contributes to the alterations in microfilament organization at the cell periphery, we have expressed fascin in LLC-PK1 epithelial cells to levels as high as those found in transformed cells and in specialized normal cells. Expression of fascin results in large changes in morphology, the actin cytoskeleton, and cell motility: fascin-transfected cells form an increased number of longer and thicker microvilli on apical surfaces, extend lamellipodia-like structures at basolateral surfaces, and show disorganization of cell-cell contacts. Cell migration activity is increased by 8-17 times when assayed by modified Boyden chamber. Microinjection of a fascin protein into LLC-PK1 cells causes similar morphological alterations including the induction of lamellipodia at basolateral surfaces and formation of an increased number of microvilli on apical surfaces. Furthermore, microinjection of fascin into REF-52 cells, normal fibroblasts, induces the formation of many lamellipodia at all regions of cell periphery. These results together suggest that fascin is directly responsible for membrane protrusions through reorganization of the microfilament cytoskeleton at the cell periphery.
Figures
Figure 1
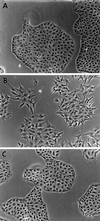
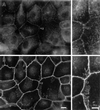
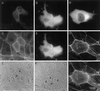
Phase contrast images of LLC-PK1, fascin-transfected, and mock-transfected cells. (A) Parental LLC-PK1 pig epithelial cells; (B) fascin-transfected cells; (C) mock-transfected cells. Note that transfected cells show many membrane extensions and that cell–cell contacts are disorganized. Bar, 100 μm.
Figure 2
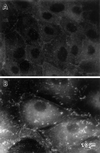
Formation of membrane extensions and microspikes by fascin transfection. Parental LLC-PK1 and fascin-transfected cells were stained with FITC-phalloidin to examine F-actin organization at the periphery. (A) Image of parental LLC-PK1 cells; (B) image of fascin-transfected cells taken at a lower focal plane; (C) image of fascin-transfected cells taken at a higher focal plane. Note that fascin transfection induces the membrane extension at lower surfaces (arrowhead in B), as well as the formation of long and thick microspikes on upper surfaces (asterisk in C). Circumferential actin bands of parental LLC-PK1 cells appear to transform into ring-like structure (arrowhead in C) from which microfilament bundles are radiating. Bar, 10 μm.
Figure 3
Disorganization of cell–cell contacts shown by fascin transfectants. Cells were double-labeled with fluorescent phalloidin and anti-β-catenin antibody. (A and B) LLC-PK1 cells; (C and D) fascin-transfected cells. (A and C) F-actin localization by phalloidin staining; (B and D) β-catenin localization. Note that cell–cell contacts are disorganized in fascin transfectants. The “rings” of transfectants (arrowhead in C and D) are stained with both the β-catenin antibody and phalloidin, confirming that they are derived from circumferential actin bands as a result of disorganization of cell–cell contacts of parental LLC-PK1 cells. Bar, 10 μm.
Figure 4
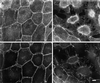
Localization of E-cadherin in LLC-PK1 and fascin transfectants. (A) LLC-PK1 cells; (B) fascin-transfected cells. The organization of E-cadherin is also disrupted. Bar, 10 μm.
Figure 5
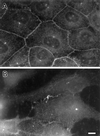
Organization of fascin and β-catenin in parental LLC-PK1 cells. (A and C) Fascin localization; (B and D) β-catenin localization. (C) and (D) are high magnification images corresponding to (A) and (B), respectively. Note that fascin is localized in short microspikes radiating from the adhesion belts while β-catenin is continuously localized on the adhesion belts (arrowhead). Bar, 10 μm.
Figure 6
Localization of fascin and β-catenin in fascin-transfected cells. Intact (A and B) or Triton-permealized (C and D) fascin transfectants were double labeled with antifascin and anti-β-catenin antibodies. (A and C) fascin; (B and D) β-catenin. In intact cells (A and B), fascin staining is not entirely diffuse but appears particulate (A). In addition, fascin shows the localization in membrane extensions (arrowhead in A). Triton permealization makes fascin’s localization in microvilli and membrane protrusions very clear (C). Fascin localizes in long and thick microvilli (arrowhead in C) radiating from “rings,” as well as in membrane extensions (arrow in C). Note that less β-catenin is found where more fascin microfilament bundles are developed (arrowhead with asterisk in C and D). Bar, 10 μm.
Figure 7
Vinculin staining of LLC-PK1 and fascin-transfected cells. (A) LLC-PK1; (B) fascin-transfectants. More and larger focal contacts are seen in transfected cells. Bar, 10 μm
Figure 8
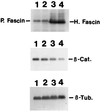
Decrease in the β-catenin expression in fascin-transfected cells. Total cell lysates of parental LLC-PK1 (lane 1), mock-transfected LLC-PK1 (lane 2), and two clones (lane 3, clone 1; lane 4, clone 2) of fascin transfectants were immunoblotted with antibodies against fascin, β-catenin, or β-tubulin. Fascin antibody (55K-2) detected both endogenous pig fascin, as well as exogenous human fascin as indicated. Clones 1 and 2 express human fascin 12 times and 17 times more than the level of endogenous fascin. In contrast, β-catenin expression of clones 1 and 2 is decreased to 33% and 20%, respectively. Mock-transfected cells show no decrease in β-catenin expression. β-tubulin level was used as an internal control. H, Human fascin; P, pig fascin; β-Cat., β-catenin; β-Tub, β-tubulin.
Figure 9
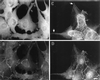
Microinjection of fascin into LLC-PK1 cells causes morphological alterations similar to those found in DNA transfection. Asterisks indicate microinjected cells. (A–C) Induction of microvilli on apical surfaces. Two LLC-PK1 cells were microinjected with human fascin, fixed with formaldehyde, and stained with rhodamin phalloidin (B) and the 55k-2 fascin antibody (A). (C) Phase contrast. The microinjected cells develop more microvilli on their apical surfaces (B). Note that longer microspikes are found radiating from circumferential bands (arrowheads). Fascin staining in (A) is not of good quality; it is only for the identification of injected cells because the fascin antibody (55k-2) does not work well with formaldehyde-fixed cells and gives a weak staining with nonspecific background. (D–F) Induction of membrane protrusions at basolateral surfaces by fascin microinjection. Four LLC-PK1 cells were microinjected, fixed with methanol, stained with the fascin antibody, and photographed at two different focal planes. (D) Basolateral surface; (E) apical surface; (F) phase contrast. Note that microinjected cells extend basolateral membranes (indicated by arrowheads in D). (G–I) Increase in phalloidin staining by microinjection with fascin. A cell was injected with human fascin together with FITC-dextran, stained with rhodamin phalloidin, and photographed using an AT200-cooled CCD camera (Photometrics). Deconvoluted images of panels H and I were obtained using MicroTome software. (G) FITC-dextran; (H) F-actin organization at a higher focal plane; (I) F-actin at a lower focal plane. F-actin staining is higher with injected cells (asterisk, H and I). Note that circumferential actin bands are partially disorganized (H, indicated by arrowhead) and that microvilli are more developed in the injected cell (H). Bar, 10 μm.
Figure 10
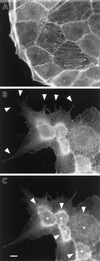
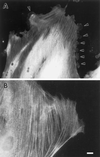
Induction of membrane protrusions by microinjection of fascin into REF-52 cells. (A) Recombinant human fascin was microinjected into REF-52 fibroblastic cells and stained with fascin antibody. (B) As a control, caldesmon was injected and stained with caldesmon antibody. Note that fascin microinjection causes numerous protrusions along all sides of the cell periphery of REF-52 cells (arrowhead in A). Bar, 10 μm.
Similar articles
- Microinjection of villin into cultured cells induces rapid and long-lasting changes in cell morphology but does not inhibit cytokinesis, cell motility, or membrane ruffling.
Franck Z, Footer M, Bretscher A. Franck Z, et al. J Cell Biol. 1990 Dec;111(6 Pt 1):2475-85. doi: 10.1083/jcb.111.6.2475. J Cell Biol. 1990. PMID: 2277069 Free PMC article. - C-erbB-2/ HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines.
Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW Jr, Yu D, Mooney EE, McCrea PD. Grothey A, et al. Oncogene. 2000 Oct 5;19(42):4864-75. doi: 10.1038/sj.onc.1203838. Oncogene. 2000. PMID: 11039904 - Roles of fascin in cell adhesion and motility.
Adams JC. Adams JC. Curr Opin Cell Biol. 2004 Oct;16(5):590-6. doi: 10.1016/j.ceb.2004.07.009. Curr Opin Cell Biol. 2004. PMID: 15363811 Review. - Fascin protrusions in cell interactions.
Adams JC. Adams JC. Trends Cardiovasc Med. 2004 Aug;14(6):221-6. doi: 10.1016/j.tcm.2004.06.002. Trends Cardiovasc Med. 2004. PMID: 15451513 Review.
Cited by
- Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis.
MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, El Agha E, Klepetko W, Seeger W, Schermuly R, Günther A, Bellusci S. MacKenzie B, et al. Respir Res. 2015 Jul 3;16(1):83. doi: 10.1186/s12931-015-0242-2. Respir Res. 2015. PMID: 26138239 Free PMC article. - Fascin-positive dendritic cells and fibroblastic reticulum cells build a framework of T-cell areas in lymph nodes.
Hiroi M, Kuroda N, Toi M, Hayashi Y, Miyazaki E, Naruse K, Enzan H. Hiroi M, et al. Virchows Arch. 2004 Feb;444(2):158-63. doi: 10.1007/s00428-003-0939-3. Epub 2003 Dec 23. Virchows Arch. 2004. PMID: 14691722 - Defining the proteomic landscape of cultured macrophages and their polarization continuum.
Oates TC, Moura PL, Cross S, Roberts K, Baum HE, Haydn-Smith KL, Wilson MC, Heesom KJ, Severn CE, Toye AM. Oates TC, et al. Immunol Cell Biol. 2023 Nov-Dec;101(10):947-963. doi: 10.1111/imcb.12687. Epub 2023 Sep 11. Immunol Cell Biol. 2023. PMID: 37694300 Free PMC article. - Epigenetic regulation of glial fibrillary acidic protein by DNA methylation in human malignant gliomas.
Restrepo A, Smith CA, Agnihotri S, Shekarforoush M, Kongkham PN, Seol HJ, Northcott P, Rutka JT. Restrepo A, et al. Neuro Oncol. 2011 Jan;13(1):42-50. doi: 10.1093/neuonc/noq145. Epub 2010 Nov 12. Neuro Oncol. 2011. PMID: 21075782 Free PMC article. - Measuring molecular rupture forces between single actin filaments and actin-binding proteins.
Ferrer JM, Lee H, Chen J, Pelz B, Nakamura F, Kamm RD, Lang MJ. Ferrer JM, et al. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9221-6. doi: 10.1073/pnas.0706124105. Epub 2008 Jun 30. Proc Natl Acad Sci U S A. 2008. PMID: 18591676 Free PMC article.
References
- Adams JC. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J Cell Sci. 1995;108:1977–1990. - PubMed
- Colombo R, DalleDonne I, Milzani A. Alpha-actinin increases actin filament end concentration by inhibiting annealing. J Mol Biol. 1993;230:1151–1158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous