Molecular and genetic analysis of two closely linked genes that encode, respectively, a protein phosphatase 1/2A/2B homolog and a protein kinase homolog in the cyanobacterium Anabaena sp. strain PCC 7120 - PubMed (original) (raw)
Molecular and genetic analysis of two closely linked genes that encode, respectively, a protein phosphatase 1/2A/2B homolog and a protein kinase homolog in the cyanobacterium Anabaena sp. strain PCC 7120
C C Zhang et al. J Bacteriol. 1998 May.
Abstract
Reversible protein phosphorylation plays important roles in signal transduction. One gene, prpA, encoding a protein similar to eukaryotic types of phosphoprotein phosphatases PP1, PP2A, and PP2B, was cloned from the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. Interestingly, a eukaryotic-type protein kinase gene, pknE, was found 301 bp downstream of prpA. This unusual genetic arrangement provides the opportunity for study about how the balance between protein phosphorylation and dephosphorylation can regulate cellular activities. Both proteins were overproduced in Escherichia coli and used to raise polyclonal antibodies. Immunodetection and RNA/DNA hybridization experiments suggest that these two genes are unlikely to be coexpressed, despite their close genetic linkage. PrpA is expressed constitutively under different nitrogen conditions, while PknE expression varies according to the nature of the nitrogen source. Inactivation analysis in vivo suggests that PrpA and PknE function to ensure a correct level of phosphorylation of the targets in order to regulate similar biological processes such as heterocyst structure formation and nitrogen fixation.
Figures
FIG. 1
Restriction map of the prpA-pknE region. The coding regions of the two genes are shown by arrow bars below the restriction map. Shaded regions correspond to the catalytic domains of PrpA and PknE. DNA fragments (pHE10, pPPE3, pp-1, and pk-1) relevant to the text are also shown. The strategies used to inactivate either the prpA gene or the pknE gene are illustrated above the restriction map (for details, see Materials and Methods).
FIG. 2
Amino acid sequence comparison between PrpA and Ser/Thr phosphatases (A) and PknE and Ser/Thr kinases (B). Identical residues are in bold-faced type. Gaps (indicated by dashes) were introduced to optimize sequence alignment. PP1-AT, protein phosphatase 1 in A. thaliana (23); PP2A-hu, human protein phosphatase 2A (25); PP2B-DM, protein phosphatase 2B in D. melanogaster (15); PknA, protein kinase in Anabaena (29); Raf-rat, the Ser/Thr kinase Raf in rats (18).
FIG. 3
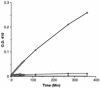
Hydrolysis of pNPP by the recombinant GST-PrpA fusion protein. The affinity-column-purified proteins (GST and GST-PrpA fusion) were incubated at 30°C in the presence of different ions. The hydrolysis of pNPP was followed by measurement of the change in OD410 (22). ⊡, GST-PrpA with 2 mM Mn2+;  , GST with 2 mM Mn2+;
, GST with 2 mM Mn2+;  , GST-PrpA with 2 mM Ca2+; and ▵, GST-PrpA with 2 mM Mg2+.
, GST-PrpA with 2 mM Ca2+; and ▵, GST-PrpA with 2 mM Mg2+.
FIG. 4
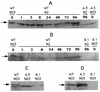
Expression of PrpA and PknE examined by Western blotting. Total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto Immobilon membrane for Western analysis with antibodies against either PrpA (A and C) or PknE (B and D). To check the protein expression during heterocyst development (A and B), similar amounts of total proteins were loaded onto each lane of the gel. Protein samples were prepared from cells grown in the presence of nitrate (lanes 0, NO3) or transferred to nitrate-free medium (N2) for 1 to 96 h (lanes 1 to lanes 96).
FIG. 5
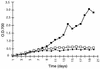
Growth curves of the wild type (▪), the prpA mutant A.3 (□), and the pknE mutant 8.1 (⧫) under N2-fixing conditions. Cells were incubated in a combined nitrogen-free medium (BG110) at 30°C in air. Cell growth was monitored by measuring the OD700.
Similar articles
- PrpJ, a PP2C-type protein phosphatase located on the plasma membrane, is involved in heterocyst maturation in the cyanobacterium Anabaena sp. PCC 7120.
Jang J, Wang L, Jeanjean R, Zhang CC. Jang J, et al. Mol Microbiol. 2007 Apr;64(2):347-58. doi: 10.1111/j.1365-2958.2007.05654.x. Epub 2007 Mar 19. Mol Microbiol. 2007. PMID: 17371502 - Genomic analysis of protein kinases, protein phosphatases and two-component regulatory systems of the cyanobacterium Anabaena sp. strain PCC 7120.
Wang L, Sun YP, Chen WL, Li JH, Zhang CC. Wang L, et al. FEMS Microbiol Lett. 2002 Dec 17;217(2):155-65. doi: 10.1111/j.1574-6968.2002.tb11469.x. FEMS Microbiol Lett. 2002. PMID: 12480098 - Two genes encoding protein kinases of the HstK family are involved in synthesis of the minor heterocyst-specific glycolipid in the cyanobacterium Anabaena sp. strain PCC 7120.
Shi L, Li JH, Cheng Y, Wang L, Chen WL, Zhang CC. Shi L, et al. J Bacteriol. 2007 Jul;189(14):5075-81. doi: 10.1128/JB.00323-07. Epub 2007 May 18. J Bacteriol. 2007. PMID: 17513480 Free PMC article. - ATP-binding cassette transporters of the multicellular cyanobacterium Anabaena sp. PCC 7120: a wide variety for a complex lifestyle.
Shvarev D, Maldener I. Shvarev D, et al. FEMS Microbiol Lett. 2018 Feb 1;365(4). doi: 10.1093/femsle/fny012. FEMS Microbiol Lett. 2018. PMID: 29360977 Review. - Genetic analysis of the kinome and phosphatome in cancer.
Arena S, Benvenuti S, Bardelli A. Arena S, et al. Cell Mol Life Sci. 2005 Sep;62(18):2092-9. doi: 10.1007/s00018-005-5205-1. Cell Mol Life Sci. 2005. PMID: 16132230 Free PMC article. Review.
Cited by
- Myxococcus xanthus Pph2 is a manganese-dependent protein phosphatase involved in energy metabolism.
García-Hernández R, Moraleda-Muñoz A, Castañeda-García A, Pérez J, Muñoz-Dorado J. García-Hernández R, et al. J Biol Chem. 2009 Oct 16;284(42):28720-8. doi: 10.1074/jbc.M109.015248. Epub 2009 Aug 25. J Biol Chem. 2009. PMID: 19706604 Free PMC article. - Global transcription profiles of the nitrogen stress response resulting in heterocyst or hormogonium development in Nostoc punctiforme.
Christman HD, Campbell EL, Meeks JC. Christman HD, et al. J Bacteriol. 2011 Dec;193(24):6874-86. doi: 10.1128/JB.05999-11. Epub 2011 Oct 14. J Bacteriol. 2011. PMID: 22001509 Free PMC article. - Genome-wide survey of putative serine/threonine protein kinases in cyanobacteria.
Zhang X, Zhao F, Guan X, Yang Y, Liang C, Qin S. Zhang X, et al. BMC Genomics. 2007 Oct 30;8:395. doi: 10.1186/1471-2164-8-395. BMC Genomics. 2007. PMID: 17971218 Free PMC article. - Survey, analysis and genetic organization of genes encoding eukaryotic-like signaling proteins on a cyanobacterial genome.
Zhang CC, Gonzalez L, Phalip V. Zhang CC, et al. Nucleic Acids Res. 1998 Aug 15;26(16):3619-25. doi: 10.1093/nar/26.16.3619. Nucleic Acids Res. 1998. PMID: 9685474 Free PMC article. Review. - Identification of two eukaryote-like serine/threonine kinases encoded by Chlamydia trachomatis serovar L2 and characterization of interacting partners of Pkn1.
Verma A, Maurelli AT. Verma A, et al. Infect Immun. 2003 Oct;71(10):5772-84. doi: 10.1128/IAI.71.10.5772-5784.2003. Infect Immun. 2003. PMID: 14500499 Free PMC article.
References
- Alex L A, Simon M I. Protein kinase and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. - PubMed
- Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. - PubMed
- Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci. 1996;21:407–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources