A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress - PubMed (original) (raw)
Comparative Study
A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress
B L Bearson et al. J Bacteriol. 1998 May.
Erratum in
- J Bacteriol 1998 Jul;180(14):3734
Abstract
The acid tolerance response enables Salmonella typhimurium to survive exposures to potentially lethal acidic environments. The acid stress imposed in a typical assay for acid tolerance (log-phase cells in minimal glucose medium) was shown to comprise both inorganic (i.e., low pH) and organic acid components. A gene previously determined to affect acid tolerance, atbR, was identified as pgi, the gene encoding phosphoglucoisomerase. Mutations in pgi were shown to increase acid tolerance by preventing the synthesis of organic acids. Protocols designed to separate the stresses of inorganic from organic acids revealed that the regulators sigma38 (RpoS), Fur, and Ada have major effects on tolerance to organic acid stress but only minor effects on inorganic acid stress. In contrast, the two-component regulatory system PhoP (identified as acid shock protein ASP29) and PhoQ proved to be important for tolerance to inorganic [corrected] acid stress but had little effect against organic acid stress. PhoP mutants also failed to induce four ASPs, confirming a role for this regulator in acid tolerance. Acid shock induction of PhoP appears to occur at the transcriptional level and requires the PhoPQ system. Furthermore, induction by acid occurs even in the presence of high concentrations of magnesium, the ion known to be sensed by PhoQ. These results suggest that PhoQ can sense both Mg2+ and pH. Since phoP mutants are avirulent, the low pH activation of this system has important implications concerning the pathogenesis of S. typhimurium. The involvement of four regulators, two of which are implicated in virulence, underscores the complexity of the acid tolerance stress response and further suggests that features of acid tolerance and virulence are interwoven.
Figures
FIG. 1
The influence of rpoS, pgi, and growth on citrate on the ATR. Cells were grown to mid-log phase (approximately 2 × 108 cells per ml) in minimal EG medium (pH 7.7). When indicated, E medium with citrate was used as the carbon source. Unadapted cell cultures were adjusted to pH 3, and samples were taken for viable counts at time zero and 60 min and after acid challenge. Adapted cultures were adjusted from pH 7.7 to 4.4 for 1 h and then acid challenged for 1 h at pH 3. Wild-type UK1 (SF530), rpoS::Ap (JF2690), atbR (JF2733), rpoS atbR (JF2731), and rpoS pgi (JF2955) cells were assayed. The data are a representative sample of triplicate experiments.
FIG. 2
RpoS-dependent systems are required for effective tolerance to organic acid stress. Cells (JF2690 [rpoS::_Ap_]) were grown and treated as indicated in the legend to Fig. 1. Spent, cells were grown, adapted, and challenged in the same EG medium; fresh, cells were removed from growth medium by centrifugation and resuspended in fresh EG medium already adjusted to pH 3 for challenge. In one experiment, the cells were resuspended in fresh medium made to contain 1 mM acetate (as acetic acid). The data are a representative sample of triplicate experiments.
FIG. 3
Roles of fur and ada mutations on organic versus inorganic acid stress. Cells (UK1 [wild type], JF2690 [rpoS::_Ap_], SF588 [_fur-1_], and JF3024 [_ada_]) were grown and treated essentially as indicated in the legend to Fig. 1. Acid challenges (pH 3) were conducted in the presence (+ [spent medium]) or absence (− [fresh medium]) of organic acids. Results are representative of triplicate experiments. U, unadapted; A, adapted.
FIG. 4
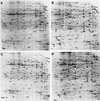
PhoP controls the acid shock induction of four ASPs. Two-dimensional SDS-PAGE analysis of ASPs produced by JF2690 (rpoS) (A and B) and JF3204 (rpoS phoP) (C and D). Unadapted cells (A and C) and cells that were shocked with acid (pH 4.4) for 20 min (B and D) are shown. PhoP-dependent ASPs are indicated by numbers in panel B and by open circles in panel D. Several other ASPs are indicated by arrowheads.
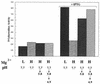
FIG. 5
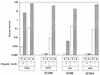
The PhoPQ two-component signal transduction system is involved in inorganic acid tolerance. Cells (indicated below each set of bars) were grown and treated as indicated in the legends to Fig. 1 and 3. U, unadapted; A, adapted.
FIG. 6
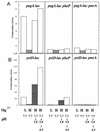
The effects of Mg2+, acid, phoP, and pmrA on the expression of pagA and psiD (pmrC). Cells were grown in N medium (pH 7.7) supplemented with 1% vitamin-free Casamino Acids to mid-log phase (2 × 108 cells/ml) in either 10 mM (H) or 10 μM (L) MgSO4. As indicated below each bar, the cells were adapted at pH 5.8 for 60 min or underwent a stepwise adaptation at pH 5.8 for 30 min followed by 30 min at pH 4.9. The results are representative of triplicate experiments. (A) pagA-lac (JF3303), pagA-lac phoP (JF3531), and pagA-lac pmrA (JF3547) cells; (B) psiD-lac (JF3550), psiD-lac phoP (JF3554), and psiD-lac pmrA (JF3561) cells.
FIG. 7
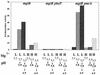
The effects of Mg2+, acid, phoP, and pmrA on the expression of mgtB. Cells were grown in N medium (pH 7.7) supplemented with 1% vitamin-free Casamino Acids to mid-log phase (2 × 108 cells/ml) in either 10 mM (H) or 10 μM (L) MgSO4. As indicated below each bar, the cells were adapted at pH 5.8 for 60 min or underwent a stepwise adaptation at pH 5.8 for 30 min followed by 30 min at pH 4.9. The results are representative of triplicate experiments. Results for mgtB-lac (JF3274), mgtB-lac phoP (JF3552), and mgtB-lac pmrA (JF3551) cells are shown.
FIG. 8
The effects of Mg2+ and acid on the expression of phoP. Cells were grown as described in the legends to Fig. 6 and 7 either in the presence or in the absence of 0.2 mM IPTG to induce the plasmid-borne phoPQ operon.
Similar articles
- Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria.
Audia JP, Webb CC, Foster JW. Audia JP, et al. Int J Med Microbiol. 2001 May;291(2):97-106. doi: 10.1078/1438-4221-00106. Int J Med Microbiol. 2001. PMID: 11437344 Review. - Acid shock induction of RpoS is mediated by the mouse virulence gene mviA of Salmonella typhimurium.
Bearson SM, Benjamin WH Jr, Swords WE, Foster JW. Bearson SM, et al. J Bacteriol. 1996 May;178(9):2572-9. doi: 10.1128/jb.178.9.2572-2579.1996. J Bacteriol. 1996. PMID: 8626324 Free PMC article. - The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium.
Lee IS, Lin J, Hall HK, Bearson B, Foster JW. Lee IS, et al. Mol Microbiol. 1995 Jul;17(1):155-67. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. Mol Microbiol. 1995. PMID: 7476202 - Inducible acid tolerance mechanisms in enteric bacteria.
Foster JW, Moreno M. Foster JW, et al. Novartis Found Symp. 1999;221:55-69; discussion 70-4. doi: 10.1002/9780470515631.ch5. Novartis Found Symp. 1999. PMID: 10207913 Review. - Activation of master virulence regulator PhoP in acidic pH requires the _Salmonella_-specific protein UgtL.
Choi J, Groisman EA. Choi J, et al. Sci Signal. 2017 Aug 29;10(494):eaan6284. doi: 10.1126/scisignal.aan6284. Sci Signal. 2017. PMID: 28851823 Free PMC article.
Cited by
- The Small RNA DsrA Influences the Acid Tolerance Response and Virulence of Salmonella enterica Serovar Typhimurium.
Ryan D, Ojha UK, Jaiswal S, Padhi C, Suar M. Ryan D, et al. Front Microbiol. 2016 Apr 26;7:599. doi: 10.3389/fmicb.2016.00599. eCollection 2016. Front Microbiol. 2016. PMID: 27199929 Free PMC article. - Role of the PhoP-PhoQ system in the virulence of Erwinia chrysanthemi strain 3937: involvement in sensitivity to plant antimicrobial peptides, survival at acid Hh, and regulation of pectolytic enzymes.
Llama-Palacios A, López-Solanilla E, Rodríguez-Palenzuela P. Llama-Palacios A, et al. J Bacteriol. 2005 Mar;187(6):2157-62. doi: 10.1128/JB.187.6.2157-2162.2005. J Bacteriol. 2005. PMID: 15743964 Free PMC article. - Phenotypic and genomic analyses of the Mycobacterium avium complex reveal differences in gastrointestinal invasion and genomic composition.
McGarvey JA, Bermudez LE. McGarvey JA, et al. Infect Immun. 2001 Dec;69(12):7242-9. doi: 10.1128/IAI.69.12.7242-7249.2001. Infect Immun. 2001. PMID: 11705893 Free PMC article. - Methylation of PhoP by CheR Regulates Salmonella Virulence.
Su Y, Li J, Zhang W, Ni J, Huang R, Wang Z, Cheng S, Wang Y, Tian Z, Zhou Q, Lin D, Wu W, Tang CM, Liu X, Lu J, Yao YF. Su Y, et al. mBio. 2021 Oct 26;12(5):e0209921. doi: 10.1128/mBio.02099-21. Epub 2021 Sep 21. mBio. 2021. PMID: 34544273 Free PMC article. - Low-pH rescue of acid-sensitive Salmonella enterica Serovar Typhi Strains by a Rhamnose-regulated arginine decarboxylase system.
Brenneman KE, Willingham C, Kong W, Curtiss R 3rd, Roland KL. Brenneman KE, et al. J Bacteriol. 2013 Jul;195(13):3062-72. doi: 10.1128/JB.00104-13. Epub 2013 May 3. J Bacteriol. 2013. PMID: 23645603 Free PMC article.
References
- Bearson S, Bearson B, Foster J W. Acid stress responses in enterobacteria. FEMS Microbiol Lett. 1997;147:173–180. - PubMed
- Foster J W. Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit Rev Microbiol. 1995;21:215–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials