Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1 - PubMed (original) (raw)
Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1
D L Tuttle et al. J Virol. 1998 Jun.
Abstract
The stage of differentiation and the lineage of CD4+ cells profoundly affect their susceptibility to infection by human immunodeficiency virus type 1 (HIV-1). While CD4(+) T lymphocytes in patients are readily susceptible to HIV-1 infection, peripheral blood monocytes are relatively resistant during acute or early infection, even though monocytes also express CD4 and viral strains with macrophage (M)-tropic phenotypes predominate. CCR5, the main coreceptor for M-tropic viruses, clearly contributes to the ability of CD4+ T cells to be infected. To determine whether low levels of CCR5 expression account for the block in infection of monocytes, we examined primary monocyte lineage cells during differentiation. Culturing of blood monocytes for 5 days led to an increase in the mean number of CCR5-positive cells from <20% of monocytes to >80% of monocyte-derived macrophages (MDM). Levels of CCR5 expression per monocyte were generally lower than those on MDM, perhaps below a minimum threshold level necessary for efficient infection. Productive infection may be restricted to the small subset of monocytes that express relatively high levels of CCR5. Steady-state CCR5 mRNA levels also increased four- to fivefold during MDM differentiation. Infection of MDM by M-tropic HIV-1JRFL resulted in >10-fold-higher levels of p24, and MDM harbored >30-fold more HIV-1 DNA copies than monocytes. In the presence of the CCR5-specific monoclonal antibody (MAb) 2D7, virus production and cellular levels of HIV-1 DNA were decreased by >80% in MDM, indicating a block in viral entry. There was a direct association between levels of CCR5 and differentiation of monocytes to macrophages. Levels of CCR5 were related to monocyte resistance and macrophage susceptibility to infection because infection by the M-tropic strain HIV-1JRFL could be blocked by MAb 2D7. These results provide direct evidence that CCR5 functions as a coreceptor for HIV-1 infection of primary macrophages.
Figures
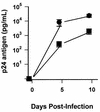
FIG. 1
HIV-1 infection of monocytes and MDM. Freshly isolated peripheral blood monocytes and MDM cultured in duplicate for 5 days were infected with HIV-1JRFL. p24 antigen levels in culture supernatants were determined for monocytes (squares) and MDM (circles) 5 and 10 days after infection. Values are the mean ± standard error of the mean for cells obtained from six uninfected donors.
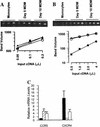
FIG. 2
Differentiation of monocytes to macrophages modulates coreceptor mRNA. Total RNA was extracted from blood monocytes or MDM after differentiation for 5 to 10 days. Relative levels of mRNAs were evaluated after synthesis of specific cDNA and amplification by a PCR titration method. Amplification of RNA carried out without reverse transcriptase never produced a product. (A) Twofold serial dilutions of cyclophilin cDNA were amplified (ethidium-stained agarose gel) and quantified (squares, monocytes; triangles, day-5 MDM; circles, day-10 MDM) to demonstrate that equal amounts of total RNA were introduced into each cDNA reaction mixture. (B) Twofold serial dilutions of CCR5 cDNA were amplified from monocytes and MDM from one individual (symbols are as in panel A). (C) CCR5 and CXCR4 mRNAs from five individuals were titrated and quantified. Mean (± standard error of the mean) levels of CCR5 in MDM after culturing for 5 days (hatched bar) or 10 days (open bar) are reported relative to levels in monocytes (solid bar). CXCR4 levels in monocytes (solid bar) and MDM cultured for 10 days (open bar) are reported relative to expression by MDM after culturing for 5 days (hatched bar).
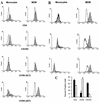
FIG. 3
Surface expression of HIV-1 receptors on monocytes and MDM. Freshly isolated PBMC and MDM cultured for 5 days were stained with MAb specific for CD4, CXCR4 (MAb 12G5), or CCR5 (MAb 2D7 and 5C7) and analyzed by two-color flow cytometry. Cells were gated on CD14 for analysis, and percentages of positive cells were calculated as described in Materials and Methods. (A) Cells obtained from subject 1. Histograms of monocytes (left panels) and MDM (right panels) for specific antibody staining (shaded histograms) are shown relative to histograms for isotype-matched, nonspecific antibody staining (open histograms). Fluorescence intensity is displayed on the x axis. (B) Monocytes and MDM from three additional individuals were stained for CD14 and CCR5 with MAb 2D7 and reported as in panel A. (C) Mean (± standard error of the mean) percentages of positive cells for peripheral blood monocytes (solid bars) and MDM after 5 days of differentiation (hatched bars) from four individuals are shown.
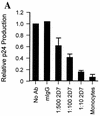
FIG. 4
CCR5 MAb 2D7 blocks infection of MDM by HIV-1JRFL. (A) Monocytes and MDM obtained from four donors and analyzed for coreceptor expression in Fig. 3 were infected in the absence or presence of MAb (Ab) 2D7 or 40 μg of mIgG per ml. Dilutions of 1:500, 1:100, or 1:10 resulted in final MAb 2D7 concentrations of 0.8, 3.9, and 39 μg/ml, respectively. Mean levels of p24 produced by monocytes or MDM infected in the presence of antibody were calculated relative to levels of p24 produced by MDM infected in the absence of antibody. In the absence of antibody MDM produced a mean of 2,939 (±1,129) pg of p24 per ml (relative to a value of 1), which was reduced to 421 (±105) pg of p24 per ml (relative to a value of <0.2) in the presence of the largest amount of MAb 2D7 (1:10). The mean p24 level produced when monocytes from the same individuals were infected was 200 ± 102 pg/ml (relative to a value of <0.1). (B) Levels of HIV-1 DNA were determined for the same monocytes (Monos) and MDM as those infected in panel A. (Left panel) DNA was extracted, and the HIV-1 env region was amplified. env products amplified from monocytes were exposed four times longer than MDM-derived products to allow visualization. β-Actin amplification served as a control for amounts of input DNA. HIV-1 DNA standard curves were analyzed in parallel with β-actin and env amplifications of 8E5 cell DNA to allow estimation of numbers of infected cells. (Right panel) Levels of env product formation in infected cells from the four donors were quantified and are reported relative to those produced by PCR amplification of DNA from cells infected in the absence of antibody.
FIG. 4
CCR5 MAb 2D7 blocks infection of MDM by HIV-1JRFL. (A) Monocytes and MDM obtained from four donors and analyzed for coreceptor expression in Fig. 3 were infected in the absence or presence of MAb (Ab) 2D7 or 40 μg of mIgG per ml. Dilutions of 1:500, 1:100, or 1:10 resulted in final MAb 2D7 concentrations of 0.8, 3.9, and 39 μg/ml, respectively. Mean levels of p24 produced by monocytes or MDM infected in the presence of antibody were calculated relative to levels of p24 produced by MDM infected in the absence of antibody. In the absence of antibody MDM produced a mean of 2,939 (±1,129) pg of p24 per ml (relative to a value of 1), which was reduced to 421 (±105) pg of p24 per ml (relative to a value of <0.2) in the presence of the largest amount of MAb 2D7 (1:10). The mean p24 level produced when monocytes from the same individuals were infected was 200 ± 102 pg/ml (relative to a value of <0.1). (B) Levels of HIV-1 DNA were determined for the same monocytes (Monos) and MDM as those infected in panel A. (Left panel) DNA was extracted, and the HIV-1 env region was amplified. env products amplified from monocytes were exposed four times longer than MDM-derived products to allow visualization. β-Actin amplification served as a control for amounts of input DNA. HIV-1 DNA standard curves were analyzed in parallel with β-actin and env amplifications of 8E5 cell DNA to allow estimation of numbers of infected cells. (Right panel) Levels of env product formation in infected cells from the four donors were quantified and are reported relative to those produced by PCR amplification of DNA from cells infected in the absence of antibody.
Similar articles
- Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains.
Huang L, Bosch I, Hofmann W, Sodroski J, Pardee AB. Huang L, et al. J Virol. 1998 Nov;72(11):8952-60. doi: 10.1128/JVI.72.11.8952-8960.1998. J Virol. 1998. PMID: 9765440 Free PMC article. - Effect of RANTES on the infection of monocyte-derived primary macrophages by human immunodeficiency virus type 1 and type 2.
Ylisastigui L, Amzazi S, Bakri Y, Vizzavona J, Vita C, Gluckman JC, Benjouad A. Ylisastigui L, et al. Biomed Pharmacother. 1998;52(10):447-53. doi: 10.1016/s0753-3322(99)80023-7. Biomed Pharmacother. 1998. PMID: 9921414 - Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates.
Li S, Juarez J, Alali M, Dwyer D, Collman R, Cunningham A, Naif HM. Li S, et al. J Virol. 1999 Dec;73(12):9741-55. doi: 10.1128/JVI.73.12.9741-9755.1999. J Virol. 1999. PMID: 10559284 Free PMC article. - The role of monocytes and macrophages in the pathogenesis of HIV-1 infection.
Kedzierska K, Crowe SM. Kedzierska K, et al. Curr Med Chem. 2002 Nov;9(21):1893-903. doi: 10.2174/0929867023368935. Curr Med Chem. 2002. PMID: 12369874 Review. - Cells of the monocyte-macrophage lineage and pathogenesis of HIV-1 infection.
Martín JC, Bandrés JC. Martín JC, et al. J Acquir Immune Defic Syndr. 1999 Dec 15;22(5):413-29. doi: 10.1097/00126334-199912150-00001. J Acquir Immune Defic Syndr. 1999. PMID: 10961602 Review.
Cited by
- Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis.
Williams DW, Eugenin EA, Calderon TM, Berman JW. Williams DW, et al. J Leukoc Biol. 2012 Mar;91(3):401-15. doi: 10.1189/jlb.0811394. Epub 2012 Jan 6. J Leukoc Biol. 2012. PMID: 22227964 Free PMC article. Review. - KLF2 in Myeloid Lineage Cells Regulates the Innate Immune Response during Skeletal Muscle Injury and Regeneration.
Manoharan P, Song T, Radzyukevich TL, Sadayappan S, Lingrel JB, Heiny JA. Manoharan P, et al. iScience. 2019 Jul 26;17:334-346. doi: 10.1016/j.isci.2019.07.009. Epub 2019 Jul 8. iScience. 2019. PMID: 31326700 Free PMC article. - Effect of cytokines on Siglec-1 and HIV-1 entry in monocyte-derived macrophages: the importance of HIV-1 envelope V1V2 region.
Jobe O, Trinh HV, Kim J, Alsalmi W, Tovanabutra S, Ehrenberg PK, Peachman KK, Gao G, Thomas R, Kim JH, Michael NL, Alving CR, Rao VB, Rao M. Jobe O, et al. J Leukoc Biol. 2016 Jun;99(6):1089-106. doi: 10.1189/jlb.2A0815-361R. Epub 2015 Dec 14. J Leukoc Biol. 2016. PMID: 26667473 Free PMC article. - Emerging immunotherapies for rheumatoid arthritis.
Reynolds G, Cooles FA, Isaacs JD, Hilkens CM. Reynolds G, et al. Hum Vaccin Immunother. 2014;10(4):822-37. doi: 10.4161/hv.27910. Epub 2014 Feb 17. Hum Vaccin Immunother. 2014. PMID: 24535556 Free PMC article. Review. - Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State.
Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, Clayton C, Austin G, Palmer BE, Zheng JH, Klein B, Kerr BJ, Guida LA, Rower C, Rower JE, Kiser JJ, Bushman LR, MaWhinney S, Anderson PL. Seifert SM, et al. AIDS Res Hum Retroviruses. 2016 Oct/Nov;32(10-11):981-991. doi: 10.1089/AID.2016.0008. Epub 2016 Sep 19. AIDS Res Hum Retroviruses. 2016. PMID: 27526873 Free PMC article.
References
- Aleixo L F, Goodenow M M, Sleasman J W. Zidovudine administered to women infected with human immunodeficiency virus type 1 and to their neonates reduces pediatric infection independent of an effect on levels of maternal virus. J Pediatr. 1997;130:906–914. - PubMed
- Aleixo, L. F., and M. M. Goodenow. Unpublished data. - PubMed
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
- Arenzana-Seisdedos F, Virelizier J-L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD32259/HD/NICHD NIH HHS/United States
- HL58005/HL/NHLBI NIH HHS/United States
- T32 CA009126/CA/NCI NIH HHS/United States
- R01 HD032259/HD/NICHD NIH HHS/United States
- R01 AI028571/AI/NIAID NIH HHS/United States
- R37 AI028571/AI/NIAID NIH HHS/United States
- AI28571/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials