In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5 - PubMed (original) (raw)
In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5
L Zhang et al. J Virol. 1998 Jun.
Abstract
We have evaluated the in vivo distribution of the major human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) coreceptors, CXCR4, CCR3, and CCR5, in both rhesus macaques and humans. T lymphocytes and macrophages in both lymphoid and nonlymphoid tissues are the major cell populations expressing HIV/SIV coreceptors, reaffirming that these cells are the major targets of HIV/SIV infection in vivo. In lymphoid tissues such as the lymph node and the thymus, approximately 1 to 10% of the T lymphocytes and macrophages are coreceptor positive. However, coreceptor expression was not detected on follicular dendritic cells (FDC) in lymph nodes, suggesting that the ability of FDC to trap extracellular virions is unlikely to be mediated by a coreceptor-specific mechanism. In the thymus, a large number of immature and mature T lymphocytes express CXCR4, which may render these cells susceptible to infection by syncytium-inducing viral variants that use this coreceptor for entry. In addition, various degrees of coreceptor expression are found among different tissues and also among different cells within the same tissues. Coreceptor-positive cells are more frequently identified in the colon than in the rectum and more frequently identified in the cervix than in the vagina, suggesting that the expression levels of coreceptors are differentially regulated at different anatomic sites. Furthermore, extremely high levels of CXCR4 and CCR3 expression are found on the neurons from both the central and peripheral nervous systems. These findings may be helpful in understanding certain aspects of HIV and SIV pathogenesis and transmission.
Figures
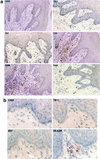
FIG. 1
(a) Immunohistochemical localization and phenotypes of coreceptor-positive cells in an ileal lymph node from rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×156 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells in the medulla are shown at two magnifications (×156 and ×468). The CCR3-positive cells are inside and surrounding the two germinal centers. The CCR5-positive cells are surrounding rather than inside the germinal centers. T lymphocytes and macrophages in the medulla are identified by the anti-CD3 and the anti-CD68 antibodies, respectively. No detectable levels of coreceptors were identified on the FDC, despite the presence of an intact FDC network in the germinal center. (b) Immunohistochemical localization and phenotypes of coreceptor-positive cells in an axillary lymph node from HIV-1-infected individual Hu1004 (AEC with hematoxylin counterstain; magnification, ×600). Some of the representative positive cells are indicated by arrowheads. The CXCR4- and CCR3-positive cells in the medulla are morphologically similar to macrophages identified by anti-CD68 antibody. A large number of T lymphocytes were identified by anti-CD3 antibody, but the CCR5-positive cells were not visualized. No detectable levels of CXCR4, CCR3, or CCR5 on the FDC were demonstrated despite the presence of an intact FDC network.
FIG. 1
(a) Immunohistochemical localization and phenotypes of coreceptor-positive cells in an ileal lymph node from rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×156 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells in the medulla are shown at two magnifications (×156 and ×468). The CCR3-positive cells are inside and surrounding the two germinal centers. The CCR5-positive cells are surrounding rather than inside the germinal centers. T lymphocytes and macrophages in the medulla are identified by the anti-CD3 and the anti-CD68 antibodies, respectively. No detectable levels of coreceptors were identified on the FDC, despite the presence of an intact FDC network in the germinal center. (b) Immunohistochemical localization and phenotypes of coreceptor-positive cells in an axillary lymph node from HIV-1-infected individual Hu1004 (AEC with hematoxylin counterstain; magnification, ×600). Some of the representative positive cells are indicated by arrowheads. The CXCR4- and CCR3-positive cells in the medulla are morphologically similar to macrophages identified by anti-CD68 antibody. A large number of T lymphocytes were identified by anti-CD3 antibody, but the CCR5-positive cells were not visualized. No detectable levels of CXCR4, CCR3, or CCR5 on the FDC were demonstrated despite the presence of an intact FDC network.
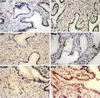
FIG. 2
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the thymus of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×100). Some of the representative positive cells are indicated by arrowheads. Due to the high density, the CXCR4-positive cells in the cortex are not as obvious as in the lymph node. The CCR3-positive cells are large and irregular compared to their neighboring cells and are believed to be macrophages identified by the anti-CD68 antibody. The majority of the CCR5-positive cells are located in the outer cortex and the central region of medulla, although a few are also identified in the interlobular septa. The epithelial framework of the thymus is stained strongly with anti-HLA-DR antibody.
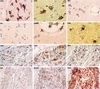
FIG. 3
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the rectum of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×154 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells are minimal and are barely visible at high magnification (×462). The CCR3- and CCR5-positive cells are located primarily in the lamina propria. A large number of T lymphocytes, macrophages, HLA-DR-positive cells, and dendritic cells are readily detected.
FIG. 4
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the colon of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×164 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells are demonstrated in the lamina propria (magnifications, ×164 and ×492). Both the CCR3- and CCR5-positive cells are shown in cross section and longitudinal section. Numerous T lymphocytes and macrophages are detected in the lamina propria. S-100 antibody avidly stains the dendritic cells in the lamina propria as well as the nerve fibers from the unnamed plexus.
FIG. 5
(a) Immunohistochemical localization and phenotypes of coreceptor-positive cells in the vagina of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×400). Some of the representative positive cells are indicated by arrowheads. No CXCR4-, CCR3-, or CCR5-positive cells were identified in the lamina propria of the vagina, despite the presence of a large number of T lymphocytes, macrophages, and dendritic cells, stained by anti-CD3, anti-CD68, and anti-S-100 antibodies, respectively. (b) Immunohistochemical localization and phenotypes of coreceptor-positive cells in the vagina of human HuVM. The arrowheads indicate some of the HLA-DR-positive cells (AEC with hematoxylin counterstain; magnification, ×600). As in rhesus macaque 1360, no CXCR4-, CCR3-, or CCR5-positive cells were identified in the lamina propria of the vagina.
FIG. 6
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the cervix of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×400). Some of the representative positive cells are indicated by arrowheads. The majority of the CXCR4- and CCR3-positive cells are believed to be macrophages or dendritic cells and are located primarily in either the lamina propria or the epithelial lining. The CCR5-positive cells are located adjacent to the epithelium. A few HLA-DR-positive cells are also located below the epithelium.
FIG. 7
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the brain (upper two panels) and in the regional ganglia (lower two panels) of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×200 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. Neurons in the brain and regional ganglia express high levels of CXCR4 (magnifications, ×400 and ×600) and CCR3 (magnification, ×400) but no detectable level of CCR5 (magnification, ×400). Macrophages located on the wall of small blood vessels (magnification, ×600) are also positive for CXCR4 (data not shown) and CCR3 (magnification, ×400). S-100 antibody stains oligodendrocytes in the CNS (magnification, ×600) and Schwann cells in the regional ganglia. Schwann cells are also positive for HLA-DR.
Similar articles
- Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells.
Zhang Y, Lou B, Lal RB, Gettie A, Marx PA, Moore JP. Zhang Y, et al. J Virol. 2000 Aug;74(15):6893-910. doi: 10.1128/jvi.74.15.6893-6910.2000. J Virol. 2000. PMID: 10888629 Free PMC article. - Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo.
Reeves JD, Hibbitts S, Simmons G, McKnight A, Azevedo-Pereira JM, Moniz-Pereira J, Clapham PR. Reeves JD, et al. J Virol. 1999 Sep;73(9):7795-804. doi: 10.1128/JVI.73.9.7795-7804.1999. J Virol. 1999. PMID: 10438870 Free PMC article. - Chemokine receptors and virus entry in the central nervous system.
Gabuzda D, Wang J. Gabuzda D, et al. J Neurovirol. 1999 Dec;5(6):643-58. doi: 10.3109/13550289909021293. J Neurovirol. 1999. PMID: 10602405 Review. - Chemokine receptors and human immunodeficiency virus infection.
Bieniasz PD, Cullen BR. Bieniasz PD, et al. Front Biosci. 1998 Jan 1;3:d44-58. doi: 10.2741/a265. Front Biosci. 1998. PMID: 9407151 Review.
Cited by
- Virus and target cell evolution in human immunodeficiency virus type 1 infection.
Mosier DE. Mosier DE. Immunol Res. 2000;21(2-3):253-8. doi: 10.1385/IR:21:2-3:253. Immunol Res. 2000. PMID: 10852125 Review. - Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope.
Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D. Ohagen A, et al. J Virol. 1999 Feb;73(2):897-906. doi: 10.1128/JVI.73.2.897-906.1999. J Virol. 1999. PMID: 9882290 Free PMC article. - Definition of the stage of host cell genetic restriction of replication of human immunodeficiency virus type 1 in monocytes and monocyte-derived macrophages by using twins.
Naif HM, Li S, Alali M, Chang J, Mayne C, Sullivan J, Cunningham AL. Naif HM, et al. J Virol. 1999 Jun;73(6):4866-81. doi: 10.1128/JVI.73.6.4866-4881.1999. J Virol. 1999. PMID: 10233948 Free PMC article. - Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides.
Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Greenhead P, et al. J Virol. 2000 Jun;74(12):5577-86. doi: 10.1128/jvi.74.12.5577-5586.2000. J Virol. 2000. PMID: 10823865 Free PMC article. - CXCR4 in Cancer and Its Regulation by PPARgamma.
Richard CL, Blay J. Richard CL, et al. PPAR Res. 2008;2008:769413. doi: 10.1155/2008/769413. PPAR Res. 2008. PMID: 18779872 Free PMC article.
References
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP1-alpha, MIP1-beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
- Bahner I, Kearns K, Coutinho S, Leonard E H, Kohn D B. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1-induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood. 1997;90:1787–1798. - PubMed
- Broder C C, Dimitrov D S, Blumenthal R, Berger E A. The block of HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s) Virology. 1993;193:483–491. - PubMed
- Burkitt H G, Young B, Heath J W. Functional histology. 3rd ed. New York, N.Y: Churchill Livingstone; 1994.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources