Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism - PubMed (original) (raw)
Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism
K T Akama et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The major pathological features of Alzheimer's disease (AD) include amyloid plaques composed primarily of the beta-amyloid (Abeta) peptide, degenerating neurons and neurofibrillary tangles, and the presence of numerous activated astrocytes and microglia. Although extensive genetic data implicate Abeta in the neurodegenerative cascade of AD, the molecular mechanisms underlying its effects on neurons and glia and the relationship between glial activation and neuronal death are not well defined. Abeta has been shown to induce glial activation, and a growing body of evidence suggests that activated glia contribute to neurotoxicity through generation of inflammatory cytokines and neurotoxic free radicals, such as nitric oxide (NO), potent sources of oxidative stress known to occur in AD. It is therefore crucial to identify specific Abeta-induced molecular pathways mediating these responses in activated glia. We report that Abeta stimulates the activation of the transcription factor NFkappaB in rat astrocytes, that NFkappaB activation occurs selectively from p65 transactivation domain 2, and that Abeta-induced NO synthase expression and NO production occur through an NFkappaB-dependent mechanism. This demonstration of how Abeta couples an intracellular signal transduction pathway involving NFkappaB to a potentially neurotoxic response provides a key mechanistic link between Abeta and the generation of oxidative damage. Our results also suggest possible molecular targets upon which to focus future drug discovery efforts for AD.
Figures
Figure 1
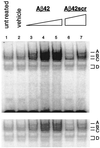
Aβ42 activates NFκB in a dose-dependent manner. Nuclear extracts from astrocytes stimulated by increasing doses of Aβ42 were incubated with 32P-labeled NFκB oligonucleotide probe for gel mobility-shift assay. Lanes: 1, untreated astrocyte nuclear extract; 2, vehicle-treated nuclear extract (0 μM Aβ42); 3–5, 1 μM, 5 μM, and 10 μM Aβ42, respectively. As a peptide control, nuclear extracts from astrocytes stimulated by a scrambled Aβ42 peptide [10 μM Aβ42scr (lane 6) and 20 μM Aβ42scr (lane 7)] were run to demonstrate the activation specificity of Aβ42. Cells were treated for 12 hr, a time-point where maximal NFκB activation could be detected by EMSA (data not shown). NFκB activation complexes are indicated by A–D. (Lower) A shorter exposure of the autoradiogram to allow better visualization of the individual complexes in lanes 4 and 5.
Figure 2
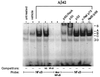
Specificity of Aβ42-activated NFκB. Gel mobility-shift assay with nuclear extracts prepared from untreated astrocytes (lane 1), vehicle-control-treated astrocytes (lane 2), and 10 μM Aβ42-treated astrocytes (lanes 3–11). Cells were incubated for 12 hr. 32P-labeled oligonucleotide shift probes used contained consensus NFκB response element (lanes 1–3 and 7–11), 50-fold molar excess of unlabeled AP2 (nonspecific competitor, NS) shift probe before 32P-labeled NFκB shift probe (lane 4), approximately 50-fold molar excess of unlabeled NFκB (specific competitor, NFκB) shift probe before 32P-labeled NFκB shift probe (lane 5), or 32P-labeled mutant NFκB carrying a 1-bp substitution within the NFκB response element (lane 6). Polyclonal antibodies used to detect the indicated Rel family subunits are as indicated (lanes 7–11). NFκB activation complexes are indicated by A–D. Supershifted complexes are indicated by S and were detected only in samples incubated with either p65/RelA or p50 antibody.
Figure 3
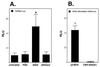
Aβ42-specific stimulation of NFκB reporter gene activation. (A) The 3xRel-LUC plasmid (three tandem NFκB response element repeats with a minimal promoter cloned upstream of the luciferase gene) was transfected into astrocytes, which were then left untreated or stimulated for 12 hr by either PBS, 10 μM Aβ42, or 10 μM Aβ42scr. Luciferase expression in Aβ42-stimulated cells was significantly increased above that in untreated, PBS-treated, or Aβ42scr-treated cells. Data shown (mean ± SEM) represent n = 8 transfections and are RLUs. (B) Cotransfecting the 3xRel-LUC construct with the IκB (Super-repressor, Sr) expression construct [CMV-IκB(Sr)] reduced Aβ-stimulated 3xRel-LUC luciferase activity to near background levels compared with cotransfection of 3xRel-LUC with the backbone vector pCMV4. ∗, Significantly different from PBS control (P < 0.05); ‡, significantly different from control vector (P < 0.005). Statistics here and results throughout have been calculated by Student’s t test. Significance is determined if P < 0.05.
Figure 4
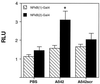
Aβ42 activates NFκB at p65/RelA TAD 2. NFκB(1)-Gal4 and NFκB(2)-Gal4 expression constructs containing the first and second TADs of p65/RelA, respectively, were individually cotransfected with the yeast Gal4-UAS luciferase reporter plasmid to determine from which domain NFκB activation by Aβ42 occurred. Ten micromolar Aβ42-stimulated astrocytes showed approximately 2- to 3-fold more NFκB(2)-Gal4 luciferase activity compared with PBS-treated or 10 μM Aβ42scr-treated cells. There was no significant stimulation of NFκB(1)-Gal4 in Aβ42-treated cells compared with PBS-treated or 10 μM Aβ42scr-treated cells. ∗, Significantly different from PBS control (P < 0.005).
Figure 5
Aβ42 stimulates iNOS in astrocytes in an NFκB-dependent manner. (A) Aβ42-stimulated iNOS promoter activity as determined by luciferase activity. Astrocytes were transfected with the 7-kb iNOS promoter luciferase reporter construct [7kb(wt) iNOS-LUC] and then either left untreated or stimulated by PBS, 10 μM Aβ42, or 10 μM Aβ42scr for 12 hr. Only Aβ42 significantly stimulated iNOS promoter activity. Inactivation of the TATAA-box proximal NFκB response element in the 7-kb iNOS promoter by site-directed mutagenesis [7kb(NFκB−) iNOS-LUC] results in no significant promoter activity by Aβ42. (Inset) The same 7kb(NFκB−) iNOS-LUC RLU data with a more focused ordinate range. U, P, A, and S are untreated, PBS-, Aβ42-, and Aβ42scr-treated cells, respectively. Data (mean RLU ± SEM) represent n = 8 transfections. (B) Cotransfecting the 7-kb iNOS-LUC construct with CMV-IκB(Sr) reduced Aβ-stimulated iNOS promoter activity to near background levels compared with cotransfection of 7kb(wt) iNOS-LUC with the backbone vector pCMV4. Data (mean RLU ± SEM) represent n = 6 transfections. (C) iNOS activity was determined by measuring the production of nitrite by a modified Griess assay as described. Aβ42-stimulated nitrite production was reduced to background control levels in astrocytes transfected with CMV-IκB(Sr), but not in Aβ42-stimulated astrocytes transfected with backbone vector pCMV4 alone. (Each transfection well received a total of 750 ng of plasmid DNA. Control backbone vector transfection wells received 750 ng of pCMV4 and CMV-IκB(Sr) transfection wells received 0.75 ng of CMV-IκB(Sr) plus 749.25 ng of pCMV4.) Data (mean ± SEM) represent n = 11 transfections. ∗, Significantly different from PBS control (P < 0.05); ‡, significantly different from CMV-IκB(Sr) (P < 0.005).
Similar articles
- Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release.
Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Hu J, et al. Brain Res. 1998 Mar 2;785(2):195-206. doi: 10.1016/s0006-8993(97)01318-8. Brain Res. 1998. PMID: 9518610 - Mechanism of glial activation by S100B: involvement of the transcription factor NFkappaB.
Lam AG, Koppal T, Akama KT, Guo L, Craft JM, Samy B, Schavocky JP, Watterson DM, Van Eldik LJ. Lam AG, et al. Neurobiol Aging. 2001 Sep-Oct;22(5):765-72. doi: 10.1016/s0197-4580(01)00233-0. Neurobiol Aging. 2001. PMID: 11705636 - beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis.
Combs CK, Karlo JC, Kao SC, Landreth GE. Combs CK, et al. J Neurosci. 2001 Feb 15;21(4):1179-88. doi: 10.1523/JNEUROSCI.21-04-01179.2001. J Neurosci. 2001. PMID: 11160388 Free PMC article. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - The killing of neurons by beta-amyloid peptides, prions, and pro-inflammatory cytokines.
Chiarini A, Dal Pra I, Whitfield JF, Armato U. Chiarini A, et al. Ital J Anat Embryol. 2006 Oct-Dec;111(4):221-46. Ital J Anat Embryol. 2006. PMID: 17385278 Review.
Cited by
- Impairment of neuronal tyrosine phosphatase STEP worsens post-ischemic inflammation and brain injury under hypertensive condition.
Paramasivam P, Choi SW, Poddar R, Paul S. Paramasivam P, et al. J Neuroinflammation. 2024 Oct 22;21(1):271. doi: 10.1186/s12974-024-03227-z. J Neuroinflammation. 2024. PMID: 39438980 Free PMC article. - Neural circuit mechanisms underlying aberrantly prolonged functional hyperemia in young Alzheimer's disease mice.
Kim TA, Cruz G, Syty MD, Wang F, Wang X, Duan A, Halterman M, Xiong Q, Palop JJ, Ge S. Kim TA, et al. Mol Psychiatry. 2024 Jul 24. doi: 10.1038/s41380-024-02680-9. Online ahead of print. Mol Psychiatry. 2024. PMID: 39043843 - The impact of astrocytic NF-κB on healthy and Alzheimer's disease brains.
Jong Huat T, Camats-Perna J, Newcombe EA, Onraet T, Campbell D, Sucic JT, Martini A, Forner S, Mirzaei M, Poon W, LaFerla FM, Medeiros R. Jong Huat T, et al. Sci Rep. 2024 Jun 21;14(1):14305. doi: 10.1038/s41598-024-65248-1. Sci Rep. 2024. PMID: 38906984 Free PMC article. - CRISPR/Cas9-mediated knock-in cells of the late-onset Alzheimer's disease-risk variant, SHARPIN G186R, reveal reduced NF-κB pathway and accelerated Aβ secretion.
Asanomi Y, Kimura T, Shimoda N, Shigemizu D, Niida S, Ozaki K. Asanomi Y, et al. J Hum Genet. 2024 May;69(5):171-176. doi: 10.1038/s10038-024-01224-x. Epub 2024 Feb 13. J Hum Genet. 2024. PMID: 38351238 Free PMC article. No abstract available. - Therapeutic efficacy and promise of stem cell-derived extracellular vesicles in Alzheimer's disease and other aging-related disorders.
Rather HA, Almousa S, Craft S, Deep G. Rather HA, et al. Ageing Res Rev. 2023 Dec;92:102088. doi: 10.1016/j.arr.2023.102088. Epub 2023 Oct 11. Ageing Res Rev. 2023. PMID: 37827304 Review.
References
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–102. - PubMed
- Selkoe D J. Neuron. 1991;6:487–498. - PubMed
- Griffin W S T, Stanley L C. In: Biology and Pathology of Astrocyte-Neuron Interactions. Federoff S, editor. New York: Plenum; 1993. pp. 359–381.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources