Defective acidification in human breast tumor cells and implications for chemotherapy - PubMed (original) (raw)
Defective acidification in human breast tumor cells and implications for chemotherapy
N Altan et al. J Exp Med. 1998.
Abstract
Multidrug resistance (MDR) is a significant problem in the treatment of cancer. Chemotherapeutic drugs distribute through the cyto- and nucleoplasm of drug-sensitive cells but are excluded from the nucleus in drug-resistant cells, concentrating in cytoplasmic organelles. Weak base chemotherapeutic drugs (e.g., anthracyclines and vinca alkaloids) should concentrate in acidic organelles. This report presents a quantification of the pH for identified compartments of the MCF-7 human breast tumor cell line and demonstrates that (a) the chemotherapeutic Adriamycin concentrates in acidified organelles of drug-resistant but not drug-sensitive cells; (b) the lysosomes and recycling endosomes are not acidified in drug-sensitive cells; (c) the cytosol of drug-sensitive cells is 0.4 pH units more acidic than the cytosol of resistant cells; and (d) disrupting the acidification of the organelles of resistant cells with monensin, bafilomycin A1, or concanamycin A is sufficient to change the Adriamycin distribution to that found in drug-sensitive cells, rendering the cell vulnerable once again to chemotherapy. These results suggest that acidification of organelles is causally related to drug resistance and is consistent with the hypothesis that sequestration of drugs in acidic organelles and subsequent extrusion from the cell through the secretory pathways contribute to chemotherapeutic resistance.
Figures
Figure 1
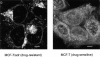
Adriamycin distribution between drug-resistant and drug-sensitive MCF-7 cells. (a) In MCF-7/ADR cells, Adriamycin is excluded from the nucleus. It is concentrated in punctate organelles throughout the cytoplasm and a brightly fluorescent region immediately adjacent to the nucleus. This perinuclear labeling is typical for the recycling endosomes and TGN. (b) In MCF-7 cells, the fluorescence of Adriamycin is localized diffusely throughout the cytoplasm and nucleoplasm, with little accumulation in any cytoplasmic compartment. Adriamycin is also seen labeling the nuclear envelope. Currently, we do not know if Adriamycin fluorescence is due to accumulation in the nuclear envelope or binding to the adjacent euchromatin. Cells were incubated in the presence of 10 μM Adriamycin as described in Materials and Methods. Scale bar, 5 μm.
Figure 2
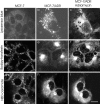
Double labeling of Adriamycin and the perinuclear recycling compartment, TGN, and highly acidified organelles. To characterize the regions that accumulated the Adriamycin in MCF-7/ADR cells, BODIPY-transferrin was used to label the recycling endosomal compartment, NBD-ceramide was used to label the TGN, and LysoSensor Blue was used to label the lysosomes. (a) MCF-7 cells accumulate very low levels of LysoSensor Blue, indicating the lack of highly acidified organelles. (b) In MCF-7/ADR cells, LysoSensor Blue labels many large punctate peripheral organelles, consistent with lysosomes. Arrows, Organelles that colabel with Adriamycin in c. (c) The same cell was subsequently labeled with 10 μM Adriamycin. Note that the perinuclear compartment, though highly labeled with Adriamycin, has few lysosomes. (d) BODIPY-transferrin labels the recycling endosome compartment, which is diffuse and punctate in the cytoplasm of MCF-7 cells. (e) BODIPY-transferrin labels the recycling endosome compartment, which is compact and polarized to one side of the nucleus in MCF-7/ADR cells. (f) Subsequent labeling of the same cells with Adriamycin also localizes in a perinuclear compartment, which overlaps the compartment labeled in e. (g) NBD-ceramide labeling of the TGN in MCF-7 cells. The TGN are distributed nonuniformly throughout the cytoplasm. In some cells, the TGN is perinuclear but not polarized to one side of the nucleus. (h) NBD-ceramide labeling of the TGN in MCF-7/ ADR cells. This compartment is compact and polarized to one side of the nucleus. (i) Subsequent labeling of the same cells with Adriamycin also localizes in a perinuclear compartment which overlaps with the TGN compartment labeled in h. Cells were either optically sectioned in 0.2-μm slices and optical sections at equivalent distances through the cell were compared, or the images were taken at a single focus which remained unchanged throughout the course of the experiments. The recycling compartment, lysosomes, and TGN were labeled as described in Materials and Methods. Scale bar, 10 μm.
Figure 3
Distribution of lysosomes in MCF-7 and MCF-7ADR cells. LAMP-1 is a membrane protein of lysosomes which is found in punctate organelles throughout the cytoplasm of (a) MCF-7/ADR cells and (b) MCF-7 cells. Analysis of large fields of MCF-7 and MCF-7/ADR cells did not reveal any significant differences in the phenotypic distribution or number of lysosomes per cell. MCF-7/ADR and MCF-7 cells were fixed in paraformaldehyde as described in Materials and Methods. Scale bar, 10 μm.
Figure 4
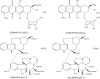
The chemical structures of four widely used chemotherapeutic drugs. Adriamycin and Daunomycin belong to the anthracycline class of compounds; vincristine and vinblastine are representative of the vinca alkaloids. Note that these drugs all are weak bases, with pKa between 7.2 and 8.4, and are all partially hydrophobic and partially hydrophilic. This property allows them to diffuse across lipid bilayers.
Figure 5
There is a lack of acidification within the subcellular compartments of drug-sensitive MCF-7 cells as assayed by acridine orange. Acridine orange is a weak base that fluoresces red when it accumulates in acidic compartments. (a) In MCF -7/ADR cells, there are many punctate red-orange fluorescing compartments throughout the cytoplasm, which is indicative of acidic organelles. (b) In MCF -7 cells, there is little red-orange fluorescence from acridine orange. This is diagnostic for few acidified organelles. Note also that the nucleus of MCF-7 cells takes up a greater amount of acridine orange than the nucleus of MCF-7/ADR cells. (c) In MCF-10F cells, a nontransformed human breast epithelial cell line, there are also many punctate red-orange fluorescing compartments distributed throughout the cytoplasm, indicative of acidic organelles. a and b were observed using confocal microscopy; c was observed under epifluorescence. Scale bar, 5 μm.
Figure 6
Specific loading of a pH probe into the cytosol of MCF-7 cells. Cells were scrape loaded with SNARF-dextran as described in Materials and Methods. Scale bar, 5 μm. (a) 70-kD SNARF-dextran loaded into MCF-7 cells. The fluorescence was excluded from the nucleoplasm and was observed as diffuse cytoplasmic fluorescence. Conjugated to a dextran, it cannot cross internal membranes. Thus, it specifically reports the pH of the cytosol. (b) MCF-7 cells were loaded with a 10-kD SNARF-dextran. The probe is present in both the cytosol and nucleoplasm. The distribution of these probes in MCF-7/ADR cells is similar. Note that in some cells, the SNARF-dextran has a punctate distribution in some areas of the cytoplasm, which may be due to an aberrant aggregation of SNARF-dextran. All pH measurements were taken from a region of cytoplasm where there was no aggregation of the probe.
Figure 7
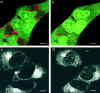
Effect of monensin on acidification and Adriamycin distribution in MCF-7/ADR cells. Monensin disrupts the acidification of subcellular compartments in drug-resistant MCF-7/ADR cells and redistributes Adriamycin to the nucleus. Scale bar, 5 μm. (a) MCF-7/ADR cells were incubated with acridine orange (6 μM) as described in the citation to Fig. 4. There is punctate red-orange fluorescence throughout the cytoplasm, indicative of acidified organelles. (b) Monensin (5 μM) was added to the solution bathing the cells in a. 30 min after addition of monensin, there was a loss of the red-orange fluorescence observed within cytoplasmic compartments. This is indicative of a loss of acidification. (c) Adriamycin was incubated with MCF-7/ ADR cells as described in the citation to Fig. 1. Adriamycin is again observed to accumulate in a perinuclear compartment that colocalizes with the lysosomes, recycling endosomes, and TGN compartments (see Fig. 2). (d) Monensin was added to the media bathing the cells in c. After 30 min, the perinuclear accumulation of Adriamycin has decreased, and instead Adriamycin is found to accumulate within the nucleus. The distribution of Adriamycin now resembles that seen in drug-sensitive MCF-7 cells (Fig. 1_b_).
Figure 8
Effect of inhibitors of the vacuolar H+-ATPase on acidification and Adriamycin distribution in MCF-7/ADR cells. Inhibitors of the vacuolar proton ATPases disrupt the acidification of drug-resistant MCF-7/ADR cells and redistribute the Adriamycin to the nucleus as assayed by laser-scanning confocal microscopy. Scale bar in c and d is 2 μM, and in a, b, and e–h is 5 μM. (a) MCF-7/ADR cells were labeled with acridine orange as in the citation to Fig. 5. The punctate red-orange fluorescence in the cytoplasm is diagnostic for acidified organelles. (b) The same cells as in a, 30 min after addition of bafilomycin A1 (500 nM). Note the disappearance of punctate red-orange cytoplasmic fluorescence, indicative of reduced acidification. (c) MCF-7/ADR cells were incubated with Adriamycin as in the citation to Fig. 1. The Adriamycin fluorescence is observed within punctate cytoplasmic organelles which colocalize with lysosomes (see Fig. 2, g–i) and with a perinuclear compartment which colocalizes with the TGN (see Fig. 2, d–f) and the recycling endosomes (see Fig. 2, a–c). (d) The same cells as in c, 30 min after addition of bafilomycin A1 (500 nM). The fluorescence of Adriamycin is decreased substantially in all cytoplasmic compartments and increased in the nucleoplasm. (e) Acridine orange–labeled MCF-7/ADR cells as in a. (f) The same cells as in e 30 min after inclusion of concanamycin (100 nM) in the incubation media. The punctate red-orange fluorescence from acridine orange accumulation is almost completely dissipated. (g) Fluorescence of Adriamycin in MCF-7/ADR cells. (h) The same cells as in g 30 min after inclusion of concanamycin (100 nM) in the incubation media. There is a substantial decrease of Adriamycin fluorescence in punctate cytoplasmic organelles and pericentriolar compartment. In contrast, there is significant increase of Adriamycin fluorescence in the nucleus.
Similar articles
- Tamoxifen inhibits acidification in cells independent of the estrogen receptor.
Altan N, Chen Y, Schindler M, Simon SM. Altan N, et al. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4432-7. doi: 10.1073/pnas.96.8.4432. Proc Natl Acad Sci U S A. 1999. PMID: 10200279 Free PMC article. - Defective pH regulation of acidic compartments in human breast cancer cells (MCF-7) is normalized in adriamycin-resistant cells (MCF-7adr).
Schindler M, Grabski S, Hoff E, Simon SM. Schindler M, et al. Biochemistry. 1996 Mar 5;35(9):2811-7. doi: 10.1021/bi952234e. Biochemistry. 1996. PMID: 8608115 - pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines.
Martínez-Zaguilán R, Raghunand N, Lynch RM, Bellamy W, Martinez GM, Rojas B, Smith D, Dalton WS, Gillies RJ. Martínez-Zaguilán R, et al. Biochem Pharmacol. 1999 May 1;57(9):1037-46. doi: 10.1016/s0006-2952(99)00022-2. Biochem Pharmacol. 1999. PMID: 10796074 - Mechanisms of altered sequestration and efflux of chemotherapeutic drugs by multidrug-resistant cells.
Ouar Z, Lacave R, Bens M, Vandewalle A. Ouar Z, et al. Cell Biol Toxicol. 1999 Apr;15(2):91-100. doi: 10.1023/a:1007521430236. Cell Biol Toxicol. 1999. PMID: 10408356 Review.
Cited by
- Functional characterization of the Plasmodium falciparum chloroquine-resistance transporter (PfCRT) in transformed Dictyostelium discoideum vesicles.
Papakrivos J, Sá JM, Wellems TE. Papakrivos J, et al. PLoS One. 2012;7(6):e39569. doi: 10.1371/journal.pone.0039569. Epub 2012 Jun 19. PLoS One. 2012. PMID: 22724026 Free PMC article. - Overcoming multidrug resistance via photodestruction of ABCG2-rich extracellular vesicles sequestering photosensitive chemotherapeutics.
Goler-Baron V, Assaraf YG. Goler-Baron V, et al. PLoS One. 2012;7(4):e35487. doi: 10.1371/journal.pone.0035487. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22530032 Free PMC article. - A role for intracellular pH in membrane IgM-mediated cell death of human B lymphomas.
Marches R, Vitetta ES, Uhr JW. Marches R, et al. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3434-9. doi: 10.1073/pnas.061028998. Epub 2001 Feb 27. Proc Natl Acad Sci U S A. 2001. PMID: 11248096 Free PMC article. - Natural product inhibitors of Hsp90: potential leads for drug discovery.
Amolins MW, Blagg BS. Amolins MW, et al. Mini Rev Med Chem. 2009 Feb;9(2):140-52. doi: 10.2174/138955709787316056. Mini Rev Med Chem. 2009. PMID: 19200020 Free PMC article. Review. - Precise detection of pH inside large unilamellar vesicles using membrane-impermeable dendritic porphyrin-based nanoprobes.
Leiding T, Górecki K, Kjellman T, Vinogradov SA, Hägerhäll C, Arsköld SP. Leiding T, et al. Anal Biochem. 2009 May 15;388(2):296-305. doi: 10.1016/j.ab.2009.02.023. Epub 2009 Feb 25. Anal Biochem. 2009. PMID: 19248752 Free PMC article.
References
- Biedler JL, Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970;30:1174–1184. - PubMed
- Ling V. Drug resistance and membrane alteration in mutants of mammalian cells. Can J Genet Cytol. 1975;17:503–515. - PubMed
- Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. - PubMed
- Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AMV, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical